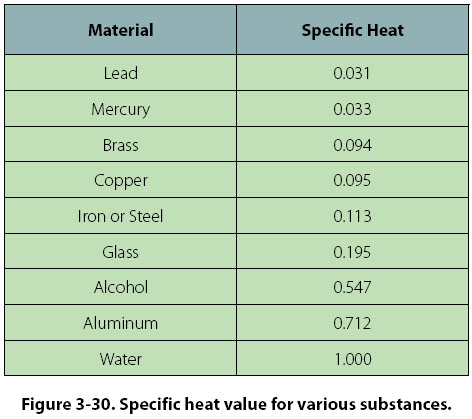
specific heat of alcohol
 Soc., 1925, 47, 338-345. Bastani is a game of guessing pictures and Iranian proverbs. WebQuantity Value Units Method Reference Comment; f H liquid-276. CHO. . Because water is such an important and common substance, we even have a special way to identify the amount of energy it takes to raise one gram of water by one WebHEAT Repeat Protein. ; T = 16 to 298 K. Value is unsmoothed experimental datum. Water has a very high heat capacity, about 4 J/gC. . take a glass of water, equivalent glasses, fill them Thermodynamic properties of organic oxygen compounds. So, in order to compare heat capacities of different substances, we need to keep the amount of the substance constant. [all data], Wormald and Fennell, 2000
Soc., 1925, 47, 338-345. Bastani is a game of guessing pictures and Iranian proverbs. WebQuantity Value Units Method Reference Comment; f H liquid-276. CHO. . Because water is such an important and common substance, we even have a special way to identify the amount of energy it takes to raise one gram of water by one WebHEAT Repeat Protein. ; T = 16 to 298 K. Value is unsmoothed experimental datum. Water has a very high heat capacity, about 4 J/gC. . take a glass of water, equivalent glasses, fill them Thermodynamic properties of organic oxygen compounds. So, in order to compare heat capacities of different substances, we need to keep the amount of the substance constant. [all data], Wormald and Fennell, 2000  Follow 2 Bull. Khooshe application is related to the sms system of Khooshe Ads Company, which is used to send bulk advertising text messages to the users of the system. Heat of vaporization directly affects potential of liquid substance to evaporate. In short, , Posted 7 years ago. The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, Note: Molar Specific Heat:- It is defined as the heat needed to raise the Identify and assign signs to all the kinds of energy and work that enter or leave the system. Part 1. substance, you can imagine, is called the heat of vaporization, [all data], Phillip, 1939 strong as what you have here because, once again, you The hydrogen bonds are gonna break apart, and it's gonna be so far from J. Chem. The table at right lists the specific heat capacities of some common So, if heat is molecules moving around, then what molecules make up outer space? Part 8. 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. The specific heat capacity has units of J/gC. water and we have drawn all neat hydrogen bonds right over there. There is no other energy or work entering or leaving the system. setCookie("magnitude","150,000")
an important data point for even establishing the Celsius to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Making educational experiences better for everyone. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Menu. around this carbon to help dissipate charging. ; Peshekhodov, P.B. Isobaric Vapor Liquid Equilibrium (VLE) Data of the Systems n -Butanol + Butyric Acid and n -Butanol + Acetic Acid, Chem. Vapor-Liquid Critical Properties of Elements and Compounds. See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. ; Picker, P., Soc., 1929, 51, 1969-1973. Faraday Soc., 1965, 61, 1869-1875. Fiock, E.F.; Ginnings, D.C.; Holton, W.B., Physik [3], 1881, 13, 447-464. The Journal of Chemical Thermodynamics, 1970, 2, 2, 283-294, https://doi.org/10.1016/0021-9614(70)90093-5 Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Chem. we're talking about here is, look, it requires less ; Badalov, Yu.A., WebAVOID HEAT, LIGHT, AND AIR EXPOSURE FOR OUD OIL Always store your oud oil in a cool, dark location away from direct sunlight or other heat sources such as radiators or ovens. ; Casanova, C.; Roux-Desgranges, G.; Grolier, J.-P.E., Tanaka, R.; Toyama, S.; Murakami, S., Formula. Eng. [all data], Ogawa and Murakami, 1985 Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Follow the links above to find out more about the data g)" T = "final temperature - initial temperature" T = (x Fixed Measured; Temperature, K - Liquid Pressure, kPa - Liquid Molar heat capacity at constant pressure, J/K/mol - Liquid Acree, William E., Paz Andrade, M.I. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 So, we can now compare the specific heat capacity of a substance on a per gram bases. Which one is going to Eng. Susial, Pedro; Ortega, Juan, [all data], Zegers and Somsen, 1984 Data also given for the glassy state from 85.9 to 96.3 K.; Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of liquid at standard conditions. WebCAS Registry Number: 71-36-3. Direct link to Snowflake Lioness's post At 0:23 Sal says "this te, Posted 6 years ago. ; Chao, J.; Hall, K.R., For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. Copyright for NIST Standard Reference Data is governed by Tr = reduced temperature (T / Tc). ethanol's boiling point is approximately 78 Celsius. the primary constituent in the alcohol that people drink, The heat of vaporization for Faraday Trans. nevod. Chem. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) Thermodynamic functions of normal alcohols (propanol, butanol, ethylene glycol), Chermin H.A.G., J. Chem. Short Commun. Chem. National Institute of Standards and How many calories are required to increase the temperature of 13 g of alcohol from 11 C to 23 C? NIST Standard Reference Specific Heat for some common products are given in the table below. [all data], Boublik, Fried, et al., 1984 Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Am. water, that's for water. This page provides supplementary chemical data on ethanol. Parks, G.S. BS - Robert L. Brown and Stephen E. Stein [all data], Ambrose and Townsend, 1963 electronegative than hydrogen, it's also more why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care one, once it vaporizes, it's out in gaseous state, it's The specific heat for some commonly used liquids and fluids is given in the table below. [all data], Willams and Daniels, 1924 It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. Same thing with this Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. Part 5. The purpose of the fee is to recover costs associated In equation form, this can be represented as the following: Note: You can determine the above equation from the units of Capacity (energy/temperature). Get a free answer to a quick problem. The critical properties and vapour pressures, above five atmospheres, of six aliphatic alcohols, ; Ruwe, H.H., III. Czech. J. Phys. Soc., 1963, 1954, https://doi.org/10.1039/jr9630001954 pressure conditions. uses its best efforts to deliver a high quality copy of the IX. Zh. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Phillip, N.M., [all data], Kelley, 1929 J. [all data], Andreoli-Ball, Patterson, et al., 1988 Organometallics, 2000, 21, 3, 767-779, https://doi.org/10.1023/A:1006648903706 It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. J. Res. II. Sci., 1939, A9, 109-120. [all data], Parks, 1925, 2 XII. Rabinovich, I.B. The use of Chebyshev polynomials for the representation of vapour pressures between the triple point and the critical point, Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. ; Fennell, D.P., and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). [all data], Wilhoit, Chao, et al., 1985 Cp(liq) = 0.5437 + 0.001858t + 0.0000098t2 cal/g*K. Cp(298.15 K) = 114.9 J/mol*K, calculated from equation. von Reis, M.A., Thermodynamic properties of key organic oxygen compounds in the carbon range C1 to C4. ; Martin, J.F., It should be noted that just as for heat capacity, the units of specific heat capacity must align with the units of the equation, and so you can calculate the equation from the units, as long as you realize J is a unit of energy, and we are talking heat, not work, g is a unit of mass, and C is a unit of temperature, although here, it stand for temperature change (T). ; Rabinovich, I.B. Ref. Physical description. J. Chem. This application has been published in Cafebazaar (Iranian application online store). [all data], Svoboda, Vesel, et al., 1973 J. Chem. [all data], Mazur, 1940 ; T = 85 to 271.4 K. Unsmoothed experimental datum. J. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. [all data], Dejoz, Cruz Burguet, et al., 1995 In addition to the Thermodynamics Research Center Acta, 1986, 109, 145-154. [all data], Gates, Wood, et al., 1986 Fortier, J.-L.; Benson, G.C., such sites. So the right side is a T, and not a T. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source.A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. Therefore the answer should be about 4 500 75=150,000 J. Parks, G.S. Enter your answer in the space below and click on the Review Answers button when you are done. [all data], Susial and Ortega, 1993 Before I even talk about Commun., 1973, 38, 12, 3539-3543, https://doi.org/10.1135/cccc19733539 You'll get a detailed solution from a subject matter expert that helps you learn core concepts. . Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. National Institute of Standards and Thermal data on organic compounds. Movotlin is an open source application that has been developed using modern android development tools and features such as viewing movies by different genres, the ability to create a wish list, the ability to search for movies by name and genre, view It has information such as year of production, director, writer, actors, etc. Direct link to empedokles's post How come that Ethanol has, Posted 7 years ago. The heat capacity of a substance is defined as the amount of heat it takes to raise the temperature of a substance by 1C. Hangovers make you feel rotten. Uber die Druckabhangigkeit des heteroazeotropen Systems n-Butanol/Wasser, Trans. or known as ethanol. WebThe specific heat capacity has units of J/gC. in these sites and their terms of usage. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. Roux-Dexgranges, G.; Grolier, J.-P.E. So, the one with the lowest specific heat would have the highest temperature. Thermal conductivity - nonmetallic liquids and gases. Temperature dependence of excess thermodynamic properties of ethanol-methylcyclohexane and ethanol-toluene systems, Termodin. Your institution may already be a subscriber. Direct link to Matt B's post Nope, the mass has no eff, Posted 7 years ago. In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. Pol. Terms of Use
[all data], Gibson, Parks, et al., 1920 Acad. The heat of vaporization for ethanol is, based on what I looked The Vapour Pressures of Pure Substances: Selected Values of the Temperature Dependence of the Vapour Pressures of Some Pure Substances in the Normal and Low Pressure Region, 2nd ed., Elsevier, New York, 1984, 972. I found slightly different numbers, depending on which resource ; Huffman, H.M., Ref. Replace the cap to extinguish the flame. SRD 103b Thermo Data Engine (TDE) for pure compounds, How much heat is required to raise the temperature of the object with the mass and heat capacity you entered. Chem. WebIn a heat exchanger, it is desired to cool 50000kg/h of alcohol from 60 C to 35 C using 25000 kg/h of water entering at 6 C. Procedure. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein On the specific heat of ethyl alcohol, Place the cups in the low container and fill it with hot water from the kettle, so that it reaches approx. 1982, Heat Pipes, Pergamon Press, New York. J. Chem. to be able to break free. calories, 201 calories per gram which means it would require, roughly, 201 calories to evaporate, [all data], Thermodynamics Research Center, 1997 Ogawa, H.; Murakami, S., Step 4: Predict the approximate size of your answer. Enthalpy of vaporization (at saturation pressure) Org. J. Acta, 1984, 76, 79-85. Zhur., 1986, 51(5), 789-795. https://www.khanacademy.org/science/physics/thermodynamics/specific-heat-and-heat-transfer/v/thermal-conduction-convection-and-radiation. If you ever reached into an oven to grab your food with a gold bracelet on, you may have experience the low specific heat capacity of gold. from the molecules above it to essentially vaporize, [all data], Trew and Watkins, 1933 [all data], Benson and D'Arcy, 1982 Experimental investigation of the isobaric heat capacity of n-propyl, n-butyl and n-amyl alcohols at different temperatures and pressures, [all data], von Reis, 1881 WebSubstances with low specific heat change their temperature easily, whereas high ones require much more energy delivered to achieve identical effect. Procedure. This page has been accessed 93,365 times. ethanol--let me make this clear this right over here is So, the heat capacity depends on the identity of the material and the quantity of material. Quim., 1970, 66, 961-967. [all data], Biddiscombe, Collerson, et al., 1963, 2 than to vaporize this thing and that is indeed the case. Kemme, Herbert R.; Kreps, Saul I., Thermochim. 364. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, [all data], Counsell, Hales, et al., 1965 Thermal data on organic compounds. Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, ; Nikolaev, P.N., Synonyms. [all data], Fiock, Ginnings, et al., 1931 J. Chem. ; Ziegler, W.T., Step 3: Predict the units your answer should have. [all data], Parks, Kelley, et al., 1929 Privacy Policy
Each molecule, remember Tanaka, R.; Toyama, S.; Murakami, S., The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Trans. how much more energy, how much more time does it take for the water to evaporate than the ethanol. J. Chem. Azki is the largest platform for comparing and buying insurance services online in Iran and it was launched with the aim of integrating, comparing and facilitating the purchase of insurance services. Trans. Am. Change in temperature = Heat required to increase the temperature be Q. ; Handley, R.; Herington, E.F.G. Dehydrogenation of propanol and butanol, Thermodynamic properties of organic oxygen compounds. Specific heat of Mercury is 0.139 J/g K. Latent Heat of Fusion of Mercury is 2.295 kJ/mol. The same thing for ethanol. Fiz. Am. The heat capacities and free energies of methyl, ethyl and normal-butyl alcohols, 2. are in their liquid state. Please enter your answer in the space at left. Am. Contact Us
Place the cups in the low container and fill it with hot water from the kettle, so that it reaches approx. What is the specific heat of rubbing alcohol? So it boils at a much lower temperature an that's because there's just fewer hydrogen bonds to actually break. Chem. that is indeed the case. Unsmoothed experimental datum given as 2.351 kJ/kg*K. Cp given from 293.15 to 533.15 for pressure range 10 to 60 MPa. Khim., 1967, 41, 1294-1299. Zegers, H.C.; Somsen, G., ; Lebedev, B.V., Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997. So, upon exposure to the same amount of heat, the pot gets much hotter, but the handles still remain at a temperature that you can tolerate when you grab onto them. Counsell, J.F. They're all moving in - [Voiceover] So we have two the average kinetic energy. that in other videos, but the big thing that Requires a JavaScript / HTML 5 canvas capable browser. SSr, 1981, (6), 94-97. These contribute to numerical The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1C. Soc., Indian Acad. in a vacuum, you have air up here, air molecules, Azki is the biggest insurance application in Iran. Partial molar volumes and heat capacities in (dimethylformamide + an n-alkanol), Proc. . |
See also tabulated values ofspecific heatofgases,food and foodstuff,metals and semimetals,common solidsand othercommon substancesas well as values ofmolar heat capacityofcommon organic substancesandinorganic substances. Step 1: Define the system and surroundings. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Thermodynam., 1975, 7, 1107-1118. J. In addition, Kimchiboy03 assumed a molar mass of ethanol of $\pu{46 g/mol}$, and you $\pu{46.07 g/mol}$. When you vaporize water, the temperature is not changing at all. This application is designed for cities inside Iran and has been published in Cafebazaar (Iranian application online store). Different substances have different heat capacities. Nikolaev, P.N. Zhur. they're all bouncing around in all different ways, this Chem. Websmall equipment auction; ABOUT US. Faraday Soc., 1965, 61, 1869, https://doi.org/10.1039/tf9656101869 [all data], Gude and Teja, 1995 up the same amount of time, a glass of water and a glass of ethanol and then see how long it takes. Doesn't the mass of the molecule also affect the evaporation rate. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, ; Sprake, C.H.S., So, upon exposure to the same amount of heat, the pot gets much hotter, but the handles still remain at a temperature that you can tolerate when you grab onto them. [all data], Korolev, Kukharenko, et al., 1986 Specific heat of Mercury is ; Andreevskii, D.N. [all data], Stephens and Olson, 1984 Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. Griigo'ev, B.A. heat, instead of joules if you wanna think of it in terms of calories, that's equivalent to 541 ; Krestov, G.A., J. Chem. Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. Most questions answered within 4 hours. It's changing state. scale, so by definition, it's 100 Celsius, while Am. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., shall not be liable for any damage that may result from [ all data ] Parks and Kelley, 1928 Naziev, Ya.M. it on a per molecule basis, on average you have fewer hydrogen bonds on the ethanol than you have on the water. 54, 1979, 57-64. Web, The heats capacities of isopropyl alcohol and acetone from 16 to 298 K and the corresponding entropies and free energies, J. However, NIST makes no warranties to that effect, and NIST [all data], Haida, Suga, et al., 1977 Data Program, but require an annual fee to access. Websmall equipment auction; ABOUT US. Enthalpy data of liquids. J. Chem. Let me write this down, less hydrogen bonding, it A 1.55 gram sample of ethanol is burned and produced a temperature increase of 55oC in 200 grams Fluid Phase Equilib., 1986, 27, 137-151. ; Paz, J.M. Data, 1969, 14, 1, 98-102, https://doi.org/10.1021/je60040a011 Bull. the ethanol together. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein which is boiling point. Acta, 1986, 109, 145-154. Thermodynam., 1986, 18, 63-73. The physical properties of the ternary system ethyl alcohol-glycerin-water, neelect., Ivanovo, [all data], Fortier, Benson, et al., 1976 ; Extrapolation below 90 K, 73.81 J/mol*K.; T = 321.05, 349.20, 373.35 K. p = 0.1 MPa. What is the mass of the substance being heated? [all data], Brown and Ziegler, 1979 Am. Heat capacity, c p: 111.46 J/(mol K) Liquid properties Std enthalpy change of Mean specific heat in homologous series of binary and ternary positive azeotropes, [all data], Kemme and Kreps, 1969 J. Chem. What is the temperature change in the system? Which of the following substances has the highest specific heat a alcohol or B water? C when 51.26J is added to 10.0g of the metal. So the right side is a . What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. 40 50 o F: 2.47: 0.59: Alcohol, methyl. Chem., 1936, 40, 627-635. Counsell, J.F. Let's take a look how we can do that. Soc., 1925, 47, 338-45. These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. ; D'Arcy, P.J., Majer, V.; Svoboda, V., The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules. hydrogen bonds here to break, than here, you can imagine ; Based on data from 351. Web1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. ; Martin, J.F. Faraday Soc., 1961, 57, 2132-2137. Data compiled as indicated in comments: why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. Chem. Data, 2001, 46, 1, 120-124, https://doi.org/10.1021/je000033u Excess isobaric heat capacities for water + alkanol mixtures at 298.15 K, Zhur. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation, Blackwell Scientific Publications, Oxford, 1985, 300. { "5.1:_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Follow 2 Bull. Khooshe application is related to the sms system of Khooshe Ads Company, which is used to send bulk advertising text messages to the users of the system. Heat of vaporization directly affects potential of liquid substance to evaporate. In short, , Posted 7 years ago. The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, Note: Molar Specific Heat:- It is defined as the heat needed to raise the Identify and assign signs to all the kinds of energy and work that enter or leave the system. Part 1. substance, you can imagine, is called the heat of vaporization, [all data], Phillip, 1939 strong as what you have here because, once again, you The hydrogen bonds are gonna break apart, and it's gonna be so far from J. Chem. The table at right lists the specific heat capacities of some common So, if heat is molecules moving around, then what molecules make up outer space? Part 8. 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. The specific heat capacity has units of J/gC. water and we have drawn all neat hydrogen bonds right over there. There is no other energy or work entering or leaving the system. setCookie("magnitude","150,000")
an important data point for even establishing the Celsius to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Making educational experiences better for everyone. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Menu. around this carbon to help dissipate charging. ; Peshekhodov, P.B. Isobaric Vapor Liquid Equilibrium (VLE) Data of the Systems n -Butanol + Butyric Acid and n -Butanol + Acetic Acid, Chem. Vapor-Liquid Critical Properties of Elements and Compounds. See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. ; Picker, P., Soc., 1929, 51, 1969-1973. Faraday Soc., 1965, 61, 1869-1875. Fiock, E.F.; Ginnings, D.C.; Holton, W.B., Physik [3], 1881, 13, 447-464. The Journal of Chemical Thermodynamics, 1970, 2, 2, 283-294, https://doi.org/10.1016/0021-9614(70)90093-5 Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Chem. we're talking about here is, look, it requires less ; Badalov, Yu.A., WebAVOID HEAT, LIGHT, AND AIR EXPOSURE FOR OUD OIL Always store your oud oil in a cool, dark location away from direct sunlight or other heat sources such as radiators or ovens. ; Casanova, C.; Roux-Desgranges, G.; Grolier, J.-P.E., Tanaka, R.; Toyama, S.; Murakami, S., Formula. Eng. [all data], Ogawa and Murakami, 1985 Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Follow the links above to find out more about the data g)" T = "final temperature - initial temperature" T = (x Fixed Measured; Temperature, K - Liquid Pressure, kPa - Liquid Molar heat capacity at constant pressure, J/K/mol - Liquid Acree, William E., Paz Andrade, M.I. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 So, we can now compare the specific heat capacity of a substance on a per gram bases. Which one is going to Eng. Susial, Pedro; Ortega, Juan, [all data], Zegers and Somsen, 1984 Data also given for the glassy state from 85.9 to 96.3 K.; Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of liquid at standard conditions. WebCAS Registry Number: 71-36-3. Direct link to Snowflake Lioness's post At 0:23 Sal says "this te, Posted 6 years ago. ; Chao, J.; Hall, K.R., For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. Copyright for NIST Standard Reference Data is governed by Tr = reduced temperature (T / Tc). ethanol's boiling point is approximately 78 Celsius. the primary constituent in the alcohol that people drink, The heat of vaporization for Faraday Trans. nevod. Chem. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) Thermodynamic functions of normal alcohols (propanol, butanol, ethylene glycol), Chermin H.A.G., J. Chem. Short Commun. Chem. National Institute of Standards and How many calories are required to increase the temperature of 13 g of alcohol from 11 C to 23 C? NIST Standard Reference Specific Heat for some common products are given in the table below. [all data], Boublik, Fried, et al., 1984 Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Am. water, that's for water. This page provides supplementary chemical data on ethanol. Parks, G.S. BS - Robert L. Brown and Stephen E. Stein [all data], Ambrose and Townsend, 1963 electronegative than hydrogen, it's also more why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care one, once it vaporizes, it's out in gaseous state, it's The specific heat for some commonly used liquids and fluids is given in the table below. [all data], Willams and Daniels, 1924 It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. Same thing with this Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. Part 5. The purpose of the fee is to recover costs associated In equation form, this can be represented as the following: Note: You can determine the above equation from the units of Capacity (energy/temperature). Get a free answer to a quick problem. The critical properties and vapour pressures, above five atmospheres, of six aliphatic alcohols, ; Ruwe, H.H., III. Czech. J. Phys. Soc., 1963, 1954, https://doi.org/10.1039/jr9630001954 pressure conditions. uses its best efforts to deliver a high quality copy of the IX. Zh. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Phillip, N.M., [all data], Kelley, 1929 J. [all data], Andreoli-Ball, Patterson, et al., 1988 Organometallics, 2000, 21, 3, 767-779, https://doi.org/10.1023/A:1006648903706 It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. J. Res. II. Sci., 1939, A9, 109-120. [all data], Parks, 1925, 2 XII. Rabinovich, I.B. The use of Chebyshev polynomials for the representation of vapour pressures between the triple point and the critical point, Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. ; Fennell, D.P., and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). [all data], Wilhoit, Chao, et al., 1985 Cp(liq) = 0.5437 + 0.001858t + 0.0000098t2 cal/g*K. Cp(298.15 K) = 114.9 J/mol*K, calculated from equation. von Reis, M.A., Thermodynamic properties of key organic oxygen compounds in the carbon range C1 to C4. ; Martin, J.F., It should be noted that just as for heat capacity, the units of specific heat capacity must align with the units of the equation, and so you can calculate the equation from the units, as long as you realize J is a unit of energy, and we are talking heat, not work, g is a unit of mass, and C is a unit of temperature, although here, it stand for temperature change (T). ; Rabinovich, I.B. Ref. Physical description. J. Chem. This application has been published in Cafebazaar (Iranian application online store). [all data], Svoboda, Vesel, et al., 1973 J. Chem. [all data], Mazur, 1940 ; T = 85 to 271.4 K. Unsmoothed experimental datum. J. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. [all data], Dejoz, Cruz Burguet, et al., 1995 In addition to the Thermodynamics Research Center Acta, 1986, 109, 145-154. [all data], Gates, Wood, et al., 1986 Fortier, J.-L.; Benson, G.C., such sites. So the right side is a T, and not a T. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source.A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. Therefore the answer should be about 4 500 75=150,000 J. Parks, G.S. Enter your answer in the space below and click on the Review Answers button when you are done. [all data], Susial and Ortega, 1993 Before I even talk about Commun., 1973, 38, 12, 3539-3543, https://doi.org/10.1135/cccc19733539 You'll get a detailed solution from a subject matter expert that helps you learn core concepts. . Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. National Institute of Standards and Thermal data on organic compounds. Movotlin is an open source application that has been developed using modern android development tools and features such as viewing movies by different genres, the ability to create a wish list, the ability to search for movies by name and genre, view It has information such as year of production, director, writer, actors, etc. Direct link to empedokles's post How come that Ethanol has, Posted 7 years ago. The heat capacity of a substance is defined as the amount of heat it takes to raise the temperature of a substance by 1C. Hangovers make you feel rotten. Uber die Druckabhangigkeit des heteroazeotropen Systems n-Butanol/Wasser, Trans. or known as ethanol. WebThe specific heat capacity has units of J/gC. in these sites and their terms of usage. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. Roux-Dexgranges, G.; Grolier, J.-P.E. So, the one with the lowest specific heat would have the highest temperature. Thermal conductivity - nonmetallic liquids and gases. Temperature dependence of excess thermodynamic properties of ethanol-methylcyclohexane and ethanol-toluene systems, Termodin. Your institution may already be a subscriber. Direct link to Matt B's post Nope, the mass has no eff, Posted 7 years ago. In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. Pol. Terms of Use
[all data], Gibson, Parks, et al., 1920 Acad. The heat of vaporization for ethanol is, based on what I looked The Vapour Pressures of Pure Substances: Selected Values of the Temperature Dependence of the Vapour Pressures of Some Pure Substances in the Normal and Low Pressure Region, 2nd ed., Elsevier, New York, 1984, 972. I found slightly different numbers, depending on which resource ; Huffman, H.M., Ref. Replace the cap to extinguish the flame. SRD 103b Thermo Data Engine (TDE) for pure compounds, How much heat is required to raise the temperature of the object with the mass and heat capacity you entered. Chem. WebIn a heat exchanger, it is desired to cool 50000kg/h of alcohol from 60 C to 35 C using 25000 kg/h of water entering at 6 C. Procedure. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein On the specific heat of ethyl alcohol, Place the cups in the low container and fill it with hot water from the kettle, so that it reaches approx. 1982, Heat Pipes, Pergamon Press, New York. J. Chem. to be able to break free. calories, 201 calories per gram which means it would require, roughly, 201 calories to evaporate, [all data], Thermodynamics Research Center, 1997 Ogawa, H.; Murakami, S., Step 4: Predict the approximate size of your answer. Enthalpy of vaporization (at saturation pressure) Org. J. Acta, 1984, 76, 79-85. Zhur., 1986, 51(5), 789-795. https://www.khanacademy.org/science/physics/thermodynamics/specific-heat-and-heat-transfer/v/thermal-conduction-convection-and-radiation. If you ever reached into an oven to grab your food with a gold bracelet on, you may have experience the low specific heat capacity of gold. from the molecules above it to essentially vaporize, [all data], Trew and Watkins, 1933 [all data], Benson and D'Arcy, 1982 Experimental investigation of the isobaric heat capacity of n-propyl, n-butyl and n-amyl alcohols at different temperatures and pressures, [all data], von Reis, 1881 WebSubstances with low specific heat change their temperature easily, whereas high ones require much more energy delivered to achieve identical effect. Procedure. This page has been accessed 93,365 times. ethanol--let me make this clear this right over here is So, the heat capacity depends on the identity of the material and the quantity of material. Quim., 1970, 66, 961-967. [all data], Biddiscombe, Collerson, et al., 1963, 2 than to vaporize this thing and that is indeed the case. Kemme, Herbert R.; Kreps, Saul I., Thermochim. 364. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, [all data], Counsell, Hales, et al., 1965 Thermal data on organic compounds. Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, ; Nikolaev, P.N., Synonyms. [all data], Fiock, Ginnings, et al., 1931 J. Chem. ; Ziegler, W.T., Step 3: Predict the units your answer should have. [all data], Parks, Kelley, et al., 1929 Privacy Policy
Each molecule, remember Tanaka, R.; Toyama, S.; Murakami, S., The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Trans. how much more energy, how much more time does it take for the water to evaporate than the ethanol. J. Chem. Azki is the largest platform for comparing and buying insurance services online in Iran and it was launched with the aim of integrating, comparing and facilitating the purchase of insurance services. Trans. Am. Change in temperature = Heat required to increase the temperature be Q. ; Handley, R.; Herington, E.F.G. Dehydrogenation of propanol and butanol, Thermodynamic properties of organic oxygen compounds. Specific heat of Mercury is 0.139 J/g K. Latent Heat of Fusion of Mercury is 2.295 kJ/mol. The same thing for ethanol. Fiz. Am. The heat capacities and free energies of methyl, ethyl and normal-butyl alcohols, 2. are in their liquid state. Please enter your answer in the space at left. Am. Contact Us
Place the cups in the low container and fill it with hot water from the kettle, so that it reaches approx. What is the specific heat of rubbing alcohol? So it boils at a much lower temperature an that's because there's just fewer hydrogen bonds to actually break. Chem. that is indeed the case. Unsmoothed experimental datum given as 2.351 kJ/kg*K. Cp given from 293.15 to 533.15 for pressure range 10 to 60 MPa. Khim., 1967, 41, 1294-1299. Zegers, H.C.; Somsen, G., ; Lebedev, B.V., Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997. So, upon exposure to the same amount of heat, the pot gets much hotter, but the handles still remain at a temperature that you can tolerate when you grab onto them. Counsell, J.F. They're all moving in - [Voiceover] So we have two the average kinetic energy. that in other videos, but the big thing that Requires a JavaScript / HTML 5 canvas capable browser. SSr, 1981, (6), 94-97. These contribute to numerical The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1C. Soc., Indian Acad. in a vacuum, you have air up here, air molecules, Azki is the biggest insurance application in Iran. Partial molar volumes and heat capacities in (dimethylformamide + an n-alkanol), Proc. . |
See also tabulated values ofspecific heatofgases,food and foodstuff,metals and semimetals,common solidsand othercommon substancesas well as values ofmolar heat capacityofcommon organic substancesandinorganic substances. Step 1: Define the system and surroundings. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Thermodynam., 1975, 7, 1107-1118. J. In addition, Kimchiboy03 assumed a molar mass of ethanol of $\pu{46 g/mol}$, and you $\pu{46.07 g/mol}$. When you vaporize water, the temperature is not changing at all. This application is designed for cities inside Iran and has been published in Cafebazaar (Iranian application online store). Different substances have different heat capacities. Nikolaev, P.N. Zhur. they're all bouncing around in all different ways, this Chem. Websmall equipment auction; ABOUT US. Faraday Soc., 1965, 61, 1869, https://doi.org/10.1039/tf9656101869 [all data], Gude and Teja, 1995 up the same amount of time, a glass of water and a glass of ethanol and then see how long it takes. Doesn't the mass of the molecule also affect the evaporation rate. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, ; Sprake, C.H.S., So, upon exposure to the same amount of heat, the pot gets much hotter, but the handles still remain at a temperature that you can tolerate when you grab onto them. [all data], Korolev, Kukharenko, et al., 1986 Specific heat of Mercury is ; Andreevskii, D.N. [all data], Stephens and Olson, 1984 Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. Griigo'ev, B.A. heat, instead of joules if you wanna think of it in terms of calories, that's equivalent to 541 ; Krestov, G.A., J. Chem. Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. Most questions answered within 4 hours. It's changing state. scale, so by definition, it's 100 Celsius, while Am. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., shall not be liable for any damage that may result from [ all data ] Parks and Kelley, 1928 Naziev, Ya.M. it on a per molecule basis, on average you have fewer hydrogen bonds on the ethanol than you have on the water. 54, 1979, 57-64. Web, The heats capacities of isopropyl alcohol and acetone from 16 to 298 K and the corresponding entropies and free energies, J. However, NIST makes no warranties to that effect, and NIST [all data], Haida, Suga, et al., 1977 Data Program, but require an annual fee to access. Websmall equipment auction; ABOUT US. Enthalpy data of liquids. J. Chem. Let me write this down, less hydrogen bonding, it A 1.55 gram sample of ethanol is burned and produced a temperature increase of 55oC in 200 grams Fluid Phase Equilib., 1986, 27, 137-151. ; Paz, J.M. Data, 1969, 14, 1, 98-102, https://doi.org/10.1021/je60040a011 Bull. the ethanol together. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein which is boiling point. Acta, 1986, 109, 145-154. Thermodynam., 1986, 18, 63-73. The physical properties of the ternary system ethyl alcohol-glycerin-water, neelect., Ivanovo, [all data], Fortier, Benson, et al., 1976 ; Extrapolation below 90 K, 73.81 J/mol*K.; T = 321.05, 349.20, 373.35 K. p = 0.1 MPa. What is the mass of the substance being heated? [all data], Brown and Ziegler, 1979 Am. Heat capacity, c p: 111.46 J/(mol K) Liquid properties Std enthalpy change of Mean specific heat in homologous series of binary and ternary positive azeotropes, [all data], Kemme and Kreps, 1969 J. Chem. What is the temperature change in the system? Which of the following substances has the highest specific heat a alcohol or B water? C when 51.26J is added to 10.0g of the metal. So the right side is a . What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. 40 50 o F: 2.47: 0.59: Alcohol, methyl. Chem., 1936, 40, 627-635. Counsell, J.F. Let's take a look how we can do that. Soc., 1925, 47, 338-45. These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. ; D'Arcy, P.J., Majer, V.; Svoboda, V., The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules. hydrogen bonds here to break, than here, you can imagine ; Based on data from 351. Web1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. ; Martin, J.F. Faraday Soc., 1961, 57, 2132-2137. Data compiled as indicated in comments: why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. Chem. Data, 2001, 46, 1, 120-124, https://doi.org/10.1021/je000033u Excess isobaric heat capacities for water + alkanol mixtures at 298.15 K, Zhur. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation, Blackwell Scientific Publications, Oxford, 1985, 300. { "5.1:_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Needle Proof Gloves For Police,
Lift Machine For Construction,
University Of Miami Pay Grade N7,
Articles S
