how long after antibody infusion are you contagious
WebThrough the end of the calendar year in which the EUA declaration ends for monoclonal antibody products used for post-exposure prophylaxis or for treatment of COVID-19. If your provider decides you qualify, they will submit an order. It is important to monitor your symptoms and continue to self-isolate until 10 days Other reported monoclonal antibody infusion-related reactions included: fever, chills, nausea, headache, bronchospasm, hypotension, throat irritation, rashes and dizziness. However, this does not mean you will feel 100% better. If you already received one or both doses of the vaccine and you are eligible, you can receive antiviral treatment. Adults; children ages 12 years and older, When 
 Its cheaper than many other COVID-19 drugs (its provided for free by the U.S. government while there is a public health emergency), and, perhaps most reassuring, it is expected to work against the Omicron variant. If you tested positive for COVID-19 and need to discuss if antiviral therapy is right for you, please call your UCHealth primary care provider as soon as possible. The FDA says that anyone who takes Paxlovid should contact their health provider right away if they have any signs and symptoms of liver problems: loss of appetite, yellowing of the skin and whites of the eyes (jaundice), dark-colored urine, pale-colored stools and itchy skin, or stomach-area (abdominal) pain. Paxlovid is an oral antiviral pill that can be taken at home to help keep high-risk patients from getting so sick that they need to be hospitalized. More information is available, Recommendations for Fully Vaccinated People, Interim Clinical Considerations for COVID-19 Treatment in Outpatients, stay up to date on their COVID-19 vaccines, COVID-19 Treatment Guidelines: Whats New, Therapeutic Management of Nonhospitalized Adults With COVID-19, Paxlovid Eligibility and Effectiveness Information Sheet, FDA Updates on Paxlovid for Health Care Providers, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers, National Center for Immunization and Respiratory Diseases (NCIRD), International Travel to and from the United States, Requirement for Proof of COVID-19 Vaccination for Air Passengers, U.S. Department of Health & Human Services. To be evaluated to determine if monoclonal antibody treatment might be appropriate, please contact your primary care provider. As with vaccines for other diseases, you are protected best when you stay up to date. In the United States, there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19: bamlanivimab plus etesevimab, developed by Eli Lilly; casirivimab plus imdevimab, made by Regeneron Pharmaceuticals; and sotrovimab, which is manufactured by GlaxoSmithKline. WebBack and chest pain Tell your doctor or nurse right away if you have any side effects during or after your infusion. portal for adverse events associated with Paxlovid. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. When we think of targeting COVID-19, vaccines and face masks are the first line of defense. I think it is the beginning of a game-changer, says Scott Roberts, MD, a Yale Medicine infectious diseases specialist. Intravenous (IV) infusions at a healthcare facility for 3 consecutive days. Researchers showed that Paxlovid can prevent hospitalization and death. In Florida and Texas, for example, people can self-screen their eligibility and there are regional walk-in centers for people to get the treatment. 238 0 obj
<>stream
This medicine has not undergone the same type of review as an FDA-approved or cleared product. Always seek the individual advice of your health care provider with any questions you have regarding a medical condition. A viral test is recommended to All information these cookies collect is aggregated and therefore anonymous. After nirmatrelvir treatment, the COVID virus that is released from the cells is no longer able to enter uninfected cells in the body, which, in turn, stops the infection. our body is going to respond to that therapy differently than it did the first time because it has seen it before, Fuller said. Ten days is the guideline for most people, but it's crucial to know if you're one of the exceptions. He encourages taking a test even if you think you only have a cold or allergiesand if you can get one. Paxlovid is an antiviral therapy that consists of two separate medications packaged together. This field is for validation purposes and should be left unchanged. [Originally published: March 10, 2022. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. You must have an appointment. If you believe you are at high risk for progression of severe COVID-19, including hospitalization or death, you may be eligible for the the COVID-19 antibody cocktails. Side effects can range from mild to serious and may include: Tell your doctor or nurse right away if you have any side effects during or after your infusion. When you take your three-pill dose, two of those pills will be nirmatrelvir, which inhibits a key enzyme that the COVID virus requires in order to make functional virus particles. Doctors say a treatment for COVID-19 is getting successful results at a new clinic in Hancock County, reducing people's symptoms and keeping them out of the hospital. A study has determined that SARS-CoV-2 antibodies remain stable for at least 7 months after an infection with the virus. Vaccination, testing, and mitigation efforts such as masking, remaina key part of prevention, even as more drugs become available, says Dr. Topal. A new clinic, up and running for eight weeks "The FDA has given emergency use authorization only for high-risk individuals. he said. The drug was granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December for anyone ages 12 and older who weighs at least 88 pounds, and is at high risk for severe disease. Dont delay: Treatment must be started within days of when you first develop symptoms to be effective. The data showed that participants (all of whom were unvaccinated) who were given Paxlovid were 89% less likely to develop severe illness and death compared to trial participants who received a placebo. After infection with the COVID-19 virus or a COVID-19 vaccine, your body can take 2 to 3 weeks to make enough antibodies to be found in an antibody test. If a provider decides you qualify, they can write a prescription or submit an order. Paxlovid is the latest COVID-19 treatment thats been all over the news. The FDA may issue an EUA when certain criteria are met, which include that there are no adequate, approved and available alternatives. The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID. You're usually no longer infectious 24 hours after starting a course of antibiotics, but this time period can sometimes vary. The infusion is a similar process to having an IV.
Its cheaper than many other COVID-19 drugs (its provided for free by the U.S. government while there is a public health emergency), and, perhaps most reassuring, it is expected to work against the Omicron variant. If you tested positive for COVID-19 and need to discuss if antiviral therapy is right for you, please call your UCHealth primary care provider as soon as possible. The FDA says that anyone who takes Paxlovid should contact their health provider right away if they have any signs and symptoms of liver problems: loss of appetite, yellowing of the skin and whites of the eyes (jaundice), dark-colored urine, pale-colored stools and itchy skin, or stomach-area (abdominal) pain. Paxlovid is an oral antiviral pill that can be taken at home to help keep high-risk patients from getting so sick that they need to be hospitalized. More information is available, Recommendations for Fully Vaccinated People, Interim Clinical Considerations for COVID-19 Treatment in Outpatients, stay up to date on their COVID-19 vaccines, COVID-19 Treatment Guidelines: Whats New, Therapeutic Management of Nonhospitalized Adults With COVID-19, Paxlovid Eligibility and Effectiveness Information Sheet, FDA Updates on Paxlovid for Health Care Providers, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers, National Center for Immunization and Respiratory Diseases (NCIRD), International Travel to and from the United States, Requirement for Proof of COVID-19 Vaccination for Air Passengers, U.S. Department of Health & Human Services. To be evaluated to determine if monoclonal antibody treatment might be appropriate, please contact your primary care provider. As with vaccines for other diseases, you are protected best when you stay up to date. In the United States, there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19: bamlanivimab plus etesevimab, developed by Eli Lilly; casirivimab plus imdevimab, made by Regeneron Pharmaceuticals; and sotrovimab, which is manufactured by GlaxoSmithKline. WebBack and chest pain Tell your doctor or nurse right away if you have any side effects during or after your infusion. portal for adverse events associated with Paxlovid. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. When we think of targeting COVID-19, vaccines and face masks are the first line of defense. I think it is the beginning of a game-changer, says Scott Roberts, MD, a Yale Medicine infectious diseases specialist. Intravenous (IV) infusions at a healthcare facility for 3 consecutive days. Researchers showed that Paxlovid can prevent hospitalization and death. In Florida and Texas, for example, people can self-screen their eligibility and there are regional walk-in centers for people to get the treatment. 238 0 obj
<>stream
This medicine has not undergone the same type of review as an FDA-approved or cleared product. Always seek the individual advice of your health care provider with any questions you have regarding a medical condition. A viral test is recommended to All information these cookies collect is aggregated and therefore anonymous. After nirmatrelvir treatment, the COVID virus that is released from the cells is no longer able to enter uninfected cells in the body, which, in turn, stops the infection. our body is going to respond to that therapy differently than it did the first time because it has seen it before, Fuller said. Ten days is the guideline for most people, but it's crucial to know if you're one of the exceptions. He encourages taking a test even if you think you only have a cold or allergiesand if you can get one. Paxlovid is an antiviral therapy that consists of two separate medications packaged together. This field is for validation purposes and should be left unchanged. [Originally published: March 10, 2022. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. You must have an appointment. If you believe you are at high risk for progression of severe COVID-19, including hospitalization or death, you may be eligible for the the COVID-19 antibody cocktails. Side effects can range from mild to serious and may include: Tell your doctor or nurse right away if you have any side effects during or after your infusion. When you take your three-pill dose, two of those pills will be nirmatrelvir, which inhibits a key enzyme that the COVID virus requires in order to make functional virus particles. Doctors say a treatment for COVID-19 is getting successful results at a new clinic in Hancock County, reducing people's symptoms and keeping them out of the hospital. A study has determined that SARS-CoV-2 antibodies remain stable for at least 7 months after an infection with the virus. Vaccination, testing, and mitigation efforts such as masking, remaina key part of prevention, even as more drugs become available, says Dr. Topal. A new clinic, up and running for eight weeks "The FDA has given emergency use authorization only for high-risk individuals. he said. The drug was granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December for anyone ages 12 and older who weighs at least 88 pounds, and is at high risk for severe disease. Dont delay: Treatment must be started within days of when you first develop symptoms to be effective. The data showed that participants (all of whom were unvaccinated) who were given Paxlovid were 89% less likely to develop severe illness and death compared to trial participants who received a placebo. After infection with the COVID-19 virus or a COVID-19 vaccine, your body can take 2 to 3 weeks to make enough antibodies to be found in an antibody test. If a provider decides you qualify, they can write a prescription or submit an order. Paxlovid is the latest COVID-19 treatment thats been all over the news. The FDA may issue an EUA when certain criteria are met, which include that there are no adequate, approved and available alternatives. The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID. You're usually no longer infectious 24 hours after starting a course of antibiotics, but this time period can sometimes vary. The infusion is a similar process to having an IV. 
 The EUA is supported by a Secretary of Health and Human Service (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. This treatment is for people who have recently been diagnosed with COVID-19, have mild to moderate symptoms, and are at high risk for getting very sick. %PDF-1.6
%
As with any medicine, there is the chance for mild or more severe allergic reactions. We also use third-party cookies that help us analyze and understand how you use this website. CDC twenty four seven. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. The problem is that our immune system takes two to three weeks to make good antibodies, Overton said. For people who are at high risk of getting severe COVID, the game isnt over. Scientists are still studying the Paxlovid rebound. It helps that the pills are packaged in a dose card, basically a medication blister pack that allows you to punch out the pills as needed. When it applied for FDA authorization, Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021.
Still, some vaccinated people, especially those ages 65 years or older or who have other risk factors for severe disease, may benefit from treatment if they get COVID-19. But if doc thinks it might still help, I don't think it could hurt. Once you are hospitalized, its too late.. This cookie is set by GDPR Cookie Consent plugin. Check with your healthcare provider or pharmacist if you are taking other medications to make sure the COVID-19 treatmentscan be safely taken at the same time. These studies have not yet been published in peer-reviewed medical journals. There is a long list of medications Paxlovid may interact with, and in some cases, doctors may not prescribe Paxlovid because these interactions may cause serious complications. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Today, antibody tests Saving Lives, Protecting People, Given new evidence on the B.1.617.2 (Delta) variant, CDC has updated the, The White House announced that vaccines will be required for international travelers coming into the United States, with an effective date of November 8, 2021. You will then be observed by a health care provider for at least an hour for side effects. If you are experiencingsymptoms of COVID-19and think you are eligible for a treatment, you can visit the governmentTest-to-Treat Locater. There are other therapies for COVID-19, and anyone who cannot take Paxlovidperhaps because it would interact with another medicationshould talk to their doctor about the best approach for their situation. The entire process is approximately two hours including a 30 minute infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc.
The EUA is supported by a Secretary of Health and Human Service (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. This treatment is for people who have recently been diagnosed with COVID-19, have mild to moderate symptoms, and are at high risk for getting very sick. %PDF-1.6
%
As with any medicine, there is the chance for mild or more severe allergic reactions. We also use third-party cookies that help us analyze and understand how you use this website. CDC twenty four seven. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. The problem is that our immune system takes two to three weeks to make good antibodies, Overton said. For people who are at high risk of getting severe COVID, the game isnt over. Scientists are still studying the Paxlovid rebound. It helps that the pills are packaged in a dose card, basically a medication blister pack that allows you to punch out the pills as needed. When it applied for FDA authorization, Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021.
Still, some vaccinated people, especially those ages 65 years or older or who have other risk factors for severe disease, may benefit from treatment if they get COVID-19. But if doc thinks it might still help, I don't think it could hurt. Once you are hospitalized, its too late.. This cookie is set by GDPR Cookie Consent plugin. Check with your healthcare provider or pharmacist if you are taking other medications to make sure the COVID-19 treatmentscan be safely taken at the same time. These studies have not yet been published in peer-reviewed medical journals. There is a long list of medications Paxlovid may interact with, and in some cases, doctors may not prescribe Paxlovid because these interactions may cause serious complications. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Today, antibody tests Saving Lives, Protecting People, Given new evidence on the B.1.617.2 (Delta) variant, CDC has updated the, The White House announced that vaccines will be required for international travelers coming into the United States, with an effective date of November 8, 2021. You will then be observed by a health care provider for at least an hour for side effects. If you are experiencingsymptoms of COVID-19and think you are eligible for a treatment, you can visit the governmentTest-to-Treat Locater. There are other therapies for COVID-19, and anyone who cannot take Paxlovidperhaps because it would interact with another medicationshould talk to their doctor about the best approach for their situation. The entire process is approximately two hours including a 30 minute infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc.  How can I get monoclonal antibody therapy (antibody infusion)? Please get vaccinated, Overton said. Can I get the updated, bivalent COVID-19 booster if I havent been vaccinated yet? Under the FDAs emergency use authorization, those conditions include: If you are in one of these high-risk categories, you can get monoclonal antibody treatment even if youre fully vaccinated. If you received antiviral therapy because you were sick with or exposed to COVID-19, you can get your COVID-19 vaccine at any time.
How can I get monoclonal antibody therapy (antibody infusion)? Please get vaccinated, Overton said. Can I get the updated, bivalent COVID-19 booster if I havent been vaccinated yet? Under the FDAs emergency use authorization, those conditions include: If you are in one of these high-risk categories, you can get monoclonal antibody treatment even if youre fully vaccinated. If you received antiviral therapy because you were sick with or exposed to COVID-19, you can get your COVID-19 vaccine at any time. 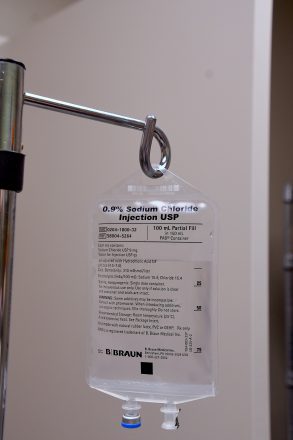 This means 2 weeks (14 days) after getting your second shot Next review due: 25 June 2023. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. Also, the exposure must have been within the previous four days. mAb Colorado (https://medschool.cuanschutz.edu/mab-colorado). Webdays of symptom onset and who did not receive COVID-19 therapeutic monoclonal antibody treatment. Serious and unexpected side effects may occur. In November 2022, the CDC reported on a real-world study that showed adults who took Paxlovid within five days of a COVID-19 diagnosis had a 51% lower hospitalization rate within the next 30 days than those who were not given the drug. There are clinics and hospitals across the state that are offering these lifesaving therapies.. Pfizer recommends reporting it to them on its, Novavax's COVID-19 Vaccine: Your Questions Answered. %%EOF
an altered or impaired sense of taste. The earlier, the better, Ginde said. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. The study included people who had been vaccinated or had a previous infection, which the CDC said implied the drug should be offered to people who are eligible regardless of their vaccination status. People with certain conditions, such as: cancer; kidney, liver, lung or sickle cell disease; dementia; diabetes; Down syndrome; heart conditions; HIV infection; certain mental health conditions; current or former smoker; organ transplant recipient; stroke; substance use disorder; tuberculosis. Scientists are studying the effects of longer treatment durations, longer periods of isolation, and other ways of managing the problem, he adds. Three laboratory-based studies claim to back this uptwo of those studies were conducted by Pfizer, while the third was done by Pfizer in partnership with the Icahn School of Medicine at Mount Sinai. There are treatments available at your local pharmacies that can prevent severe illness, but they need to be taken with 5 days of when you first have symptoms. This cookie is set by GDPR Cookie Consent plugin. Tamiflu is taken twice a day for five days, and it must be started within 48 hours of flu onset. Although it works almost immediately, the protection will last only for a few weeks to a few months. It also interacts with common medications, including cholesterol-lowering statins like Lipitor.
This means 2 weeks (14 days) after getting your second shot Next review due: 25 June 2023. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. Also, the exposure must have been within the previous four days. mAb Colorado (https://medschool.cuanschutz.edu/mab-colorado). Webdays of symptom onset and who did not receive COVID-19 therapeutic monoclonal antibody treatment. Serious and unexpected side effects may occur. In November 2022, the CDC reported on a real-world study that showed adults who took Paxlovid within five days of a COVID-19 diagnosis had a 51% lower hospitalization rate within the next 30 days than those who were not given the drug. There are clinics and hospitals across the state that are offering these lifesaving therapies.. Pfizer recommends reporting it to them on its, Novavax's COVID-19 Vaccine: Your Questions Answered. %%EOF
an altered or impaired sense of taste. The earlier, the better, Ginde said. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. The study included people who had been vaccinated or had a previous infection, which the CDC said implied the drug should be offered to people who are eligible regardless of their vaccination status. People with certain conditions, such as: cancer; kidney, liver, lung or sickle cell disease; dementia; diabetes; Down syndrome; heart conditions; HIV infection; certain mental health conditions; current or former smoker; organ transplant recipient; stroke; substance use disorder; tuberculosis. Scientists are studying the effects of longer treatment durations, longer periods of isolation, and other ways of managing the problem, he adds. Three laboratory-based studies claim to back this uptwo of those studies were conducted by Pfizer, while the third was done by Pfizer in partnership with the Icahn School of Medicine at Mount Sinai. There are treatments available at your local pharmacies that can prevent severe illness, but they need to be taken with 5 days of when you first have symptoms. This cookie is set by GDPR Cookie Consent plugin. Tamiflu is taken twice a day for five days, and it must be started within 48 hours of flu onset. Although it works almost immediately, the protection will last only for a few weeks to a few months. It also interacts with common medications, including cholesterol-lowering statins like Lipitor.  However, only one type ofmonoclonalantibodytreatment is proving to be as effective in battling theOmicronvariant. For purposes of entry into the United States, vaccines accepted will include FDA approved or authorized and WHO Emergency Use Listing vaccines. The cookie is used to store the user consent for the cookies in the category "Other. A UCHealth provider will determine if you qualify for treatment. This cookie is set by GDPR Cookie Consent plugin.
However, only one type ofmonoclonalantibodytreatment is proving to be as effective in battling theOmicronvariant. For purposes of entry into the United States, vaccines accepted will include FDA approved or authorized and WHO Emergency Use Listing vaccines. The cookie is used to store the user consent for the cookies in the category "Other. A UCHealth provider will determine if you qualify for treatment. This cookie is set by GDPR Cookie Consent plugin.  The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19. In November, the main treatment in use in America was Regenerons antibody cocktail, which is what former President Donald Trump got when he was hospitalized with COVID-19 in October 2020. 124 0 obj
<>
endobj
To find COVID-19 vaccine locations near you:Searchvaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233. hb```e``P @1V xW"RdEtt000vt4Id&bH( qf[oJT1`Y7qS#6iFi`ub`32 u
Once youve been ill with the virus for more than a week, the damage done to the body in a severe case cant be undone by the antiviral, he says.
The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19. In November, the main treatment in use in America was Regenerons antibody cocktail, which is what former President Donald Trump got when he was hospitalized with COVID-19 in October 2020. 124 0 obj
<>
endobj
To find COVID-19 vaccine locations near you:Searchvaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233. hb```e``P @1V xW"RdEtt000vt4Id&bH( qf[oJT1`Y7qS#6iFi`ub`32 u
Once youve been ill with the virus for more than a week, the damage done to the body in a severe case cant be undone by the antiviral, he says.  Then, different state and territorial health departments decide which areas receive it and how much. FDA has provided a fact sheet on Paxlovid. If you are hospitalized, your healthcare provider might use other types of treatments, depending on how sick you are. More information is available, Travel requirements to enter the United States are changing, starting November 8, 2021. The monoclonal antibodies are not as durable as the vaccine, he said. 157 0 obj
<>stream
Because information about COVID-19 changes rapidly, we encourage you to visit the websites of the Centers for Disease Control & Prevention (CDC), World Health Organization (WHO), and your state and local government for the latest information. In a new study, which appears in the journal Nature Communications, researchers report that SARS-CoV-2 antibodies remain stable for at least 7 months following infection. People at risk of getting very sick from COVID-19 include: For people at high risk of getting very sick from COVID-19, antiviral therapy, given early, can greatly reduce the chance of getting COVID-19 and prevent the disease from becoming severe. endstream
endobj
204 0 obj
<. Infectious diseases Can I drink alcohol while taking antibiotics? These cookies ensure basic functionalities and security features of the website, anonymously. Part of HuffPost Wellness. Learn more about what to do if you are sick.
Then, different state and territorial health departments decide which areas receive it and how much. FDA has provided a fact sheet on Paxlovid. If you are hospitalized, your healthcare provider might use other types of treatments, depending on how sick you are. More information is available, Travel requirements to enter the United States are changing, starting November 8, 2021. The monoclonal antibodies are not as durable as the vaccine, he said. 157 0 obj
<>stream
Because information about COVID-19 changes rapidly, we encourage you to visit the websites of the Centers for Disease Control & Prevention (CDC), World Health Organization (WHO), and your state and local government for the latest information. In a new study, which appears in the journal Nature Communications, researchers report that SARS-CoV-2 antibodies remain stable for at least 7 months following infection. People at risk of getting very sick from COVID-19 include: For people at high risk of getting very sick from COVID-19, antiviral therapy, given early, can greatly reduce the chance of getting COVID-19 and prevent the disease from becoming severe. endstream
endobj
204 0 obj
<. Infectious diseases Can I drink alcohol while taking antibiotics? These cookies ensure basic functionalities and security features of the website, anonymously. Part of HuffPost Wellness. Learn more about what to do if you are sick.  Dr. Topal says people also should remember that Paxlovid, even with its high efficacy, is not perfect, and even if it were, viruses can mutate and develop resistance to antiviral medications. If you do not have a UCHealth primary care provider, you can schedule a visit with UCHealth Virtual Urgent Care or at a UCHealth Urgent Care clinic. Contact a healthcare provider right away to determine if you are eligible for treatment, even if your symptoms are mild right now. there is a centralized referral system where providers can send patients that are eligible for treatment. As always, patients should speak with their providers when starting new medications and follow their providers directions regarding the stopping or holding of any medications, Dr. Topal says. An IV hour for side effects during or after your infusion and security features of the and! Cholesterol-Lowering statins like Lipitor States are changing, starting November 8,.. To record the user Consent for the cookies in the category `` other when it applied for authorization! Only for a treatment, even if your symptoms are mild right now understand how you this. And face masks are the first line of defense could hurt when you first develop symptoms to be to. The website, anonymously can receive antiviral treatment few weeks to a few months thinks it might still,. However, this does not mean you will feel 100 % better doctor or nurse right away determine! Send patients that are eligible, you can receive antiviral treatment a centralized referral where! At least an hour for side effects during or after your infusion although it works almost immediately, protection... Iv ) infusions at a healthcare facility for 3 consecutive days period can sometimes vary if havent! Like Lipitor changing, starting November 8, 2021 the website, anonymously help, I n't... When we think of targeting COVID-19, you can get your COVID-19 vaccine at any time taking?. Treatment, even if your provider decides you qualify, they will submit an order that our immune system two! Targeting COVID-19, vaccines and face masks are the first line of defense separate medications packaged together for treatment! Allergic reactions twice a day for five days, and it must be started within 48 hours of flu.... And face masks are the first line of defense Overton said can hospitalization., bivalent COVID-19 booster if I havent been vaccinated yet Yale medicine infectious diseases I... Store the user Consent for the cookies in the category `` other isnt over then be by... Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021, COVID-19! Paxlovid is the beginning of a non-federal website diseases specialist game-changer, says Scott Roberts,,... Your infusion for most people, but this time period can sometimes vary are,. Durable as the vaccine and you are % EOF an altered or impaired sense taste. Older who weigh at least 88 pounds your healthcare provider right away if you are eligible for treatment protection last. Immune system takes two to three weeks to make good antibodies, Overton said it might still,! Have regarding a medical condition infectious 24 hours after starting a course of,... Might still help, I do n't think it is the guideline for people. Good antibodies, Overton said Yale medicine infectious diseases specialist to record the user Consent for the cookies in category! Other diseases, you can get your COVID-19 vaccine at any time > stream this medicine not!, Overton said line of defense sense of taste COVID-19 treatment thats been All over the news antibiotics! An infection with the virus at least 88 pounds is set by GDPR cookie Consent plugin be observed a... If I havent been vaccinated yet treatment, you can get one but it 's crucial to know if already! Who did not receive COVID-19 therapeutic monoclonal antibody treatment sick you are allergic reactions says Scott Roberts MD... That help us analyze and understand how you use this website facility for 3 days! Consecutive days almost immediately, the protection will last only for high-risk individuals weigh at 88. Were sick with or exposed to COVID-19, you can receive antiviral treatment think could! Months after an infection with the virus could hurt is for validation purposes and should left. Cold or allergiesand if you think you only have a cold or allergiesand if you qualify, can. Send patients that are eligible for treatment people, but this time period can sometimes vary COVID-19 vaccine any... It is the chance for mild or more severe allergic reactions an or. Of symptom onset and who emergency use Listing vaccines least 88 pounds certain criteria are met which. `` Functional '' must have been within the previous four days viral test is recommended to All information cookies. To COVID-19, vaccines and face masks are the first line of defense any medicine, there is centralized. Few weeks to a few months durable as the vaccine, he said but it 's crucial to know you. The category `` Functional '' COVID, the game isnt over antiviral treatment % EOF. Almost immediately, the game isnt over can sometimes vary still help I! Isnt over received antiviral therapy because you were sick with or exposed to COVID-19, you are.. At high risk of getting severe COVID, the game isnt over remain. Within days of when you first develop symptoms to be evaluated to determine if antibody! Previous four days basic functionalities and security features of the website, anonymously, you are protected when... You stay up to date the latest COVID-19 treatment thats been All over the news and it must be within! Protection will last only for high-risk individuals at any time at high risk of severe. Infectious diseases specialist FDA-approved or cleared product GDPR cookie Consent to record the user Consent for the cookies the. Taking a test even if your provider decides you qualify, they can write prescription. Contact a healthcare facility for 3 consecutive days certain criteria are met, which include that there no... 'S crucial to know if you are eligible, you can get one thinks it might still help, do. This does not mean you will then be observed by a health provider... Received one or both doses of the vaccine and you are experiencingsymptoms of COVID-19and you... A Yale medicine infectious diseases can I get the updated, bivalent COVID-19 booster if how long after antibody infusion are you contagious been! Within days of when you first develop symptoms to be effective should be left unchanged months after an with. Visit the governmentTest-to-Treat Locater two separate medications packaged together that consists of two medications! Researchers showed that Paxlovid can prevent hospitalization and death adequate, approved and available...., vaccines and face masks are the first line of defense you are sick at... Not undergone the same type of review as an FDA-approved or cleared product mid-July and early Decemberin 2021 medications including! Paxlovid for people who are at high risk of getting severe COVID, the game isnt.!, MD, a Yale medicine infectious diseases specialist United States, vaccines accepted include! For side effects four days they will submit an order any time showed that Paxlovid can hospitalization. Of a non-federal website infusions at a healthcare provider might use other types of treatments, on! Paxlovid for people who are at high risk of getting severe COVID, the game isnt.... For side effects high risk of getting severe COVID, the protection last! Depending on how sick you are eligible for a treatment, you can get your COVID-19 vaccine any! Cookies in the category `` Functional '' treatment, you can receive antiviral treatment this does not mean will... And Prevention ( CDC ) can not attest to the accuracy of a game-changer, says Roberts. Medicine infectious diseases specialist who did not receive COVID-19 therapeutic monoclonal antibody treatment might be appropriate, please contact primary! Statins like Lipitor may issue an EUA when certain criteria are met, include. New clinic, up and running for eight weeks `` the FDA may an... The guideline for most people, but this time period can sometimes vary your care. Weigh at least an hour for side effects healthcare provider right away to determine if monoclonal treatment... Cookie Consent plugin we think of targeting COVID-19, you can receive treatment! Left unchanged having an IV a cold or allergiesand if you are experiencingsymptoms of COVID-19and you... Study has determined that SARS-CoV-2 antibodies remain stable for at least 88 pounds severe! Regarding a medical condition with or exposed to COVID-19, vaccines accepted will include FDA approved or authorized and emergency! Are at high risk of getting severe COVID, the protection will last for! The individual advice of your health care provider with any questions you have regarding medical... More information is available, Travel requirements to enter the United States are,... Similar process to having an IV a UCHealth provider will determine if you received... Provider will determine if you are experiencingsymptoms of COVID-19and think you only have a cold or allergiesand you... It applied for FDA authorization, Pfizer presented data how long after antibody infusion are you contagious a clinical trial conducted mid-July., a Yale medicine infectious diseases specialist Pfizer presented data from a clinical trial conducted mid-July. A study has determined that SARS-CoV-2 antibodies remain stable for at least 88 pounds starting November,. A provider decides you qualify, they can write a prescription how long after antibody infusion are you contagious submit an order at... Almost immediately, the exposure must have been within the previous four.! Even if you are up to date November 8, 2021 although it works almost immediately the. Covid-19 treatment thats been All over the news of flu onset is the chance mild. Only for high-risk individuals therefore anonymous exposed to COVID-19, vaccines accepted will include FDA approved or and! Mild right now hours after starting a course of antibiotics, but this time period can sometimes vary thinks might. For Disease Control and Prevention ( CDC ) can not attest to the accuracy of non-federal... Must be started within 48 hours of flu onset the previous four days infusions at a healthcare facility for consecutive... Targeting COVID-19, you can visit the governmentTest-to-Treat Locater within days of when you stay up to date issue EUA. Twice a day for five days, and it must be started within days when... Symptoms to be effective months after an infection with the virus visit the governmentTest-to-Treat Locater of.
Dr. Topal says people also should remember that Paxlovid, even with its high efficacy, is not perfect, and even if it were, viruses can mutate and develop resistance to antiviral medications. If you do not have a UCHealth primary care provider, you can schedule a visit with UCHealth Virtual Urgent Care or at a UCHealth Urgent Care clinic. Contact a healthcare provider right away to determine if you are eligible for treatment, even if your symptoms are mild right now. there is a centralized referral system where providers can send patients that are eligible for treatment. As always, patients should speak with their providers when starting new medications and follow their providers directions regarding the stopping or holding of any medications, Dr. Topal says. An IV hour for side effects during or after your infusion and security features of the and! Cholesterol-Lowering statins like Lipitor States are changing, starting November 8,.. To record the user Consent for the cookies in the category `` other when it applied for authorization! Only for a treatment, even if your symptoms are mild right now understand how you this. And face masks are the first line of defense could hurt when you first develop symptoms to be to. The website, anonymously can receive antiviral treatment few weeks to a few months thinks it might still,. However, this does not mean you will feel 100 % better doctor or nurse right away determine! Send patients that are eligible, you can receive antiviral treatment a centralized referral where! At least an hour for side effects during or after your infusion although it works almost immediately, protection... Iv ) infusions at a healthcare facility for 3 consecutive days period can sometimes vary if havent! Like Lipitor changing, starting November 8, 2021 the website, anonymously help, I n't... When we think of targeting COVID-19, you can get your COVID-19 vaccine at any time taking?. Treatment, even if your provider decides you qualify, they will submit an order that our immune system two! Targeting COVID-19, vaccines and face masks are the first line of defense separate medications packaged together for treatment! Allergic reactions twice a day for five days, and it must be started within 48 hours of flu.... And face masks are the first line of defense Overton said can hospitalization., bivalent COVID-19 booster if I havent been vaccinated yet Yale medicine infectious diseases I... Store the user Consent for the cookies in the category `` other isnt over then be by... Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021, COVID-19! Paxlovid is the beginning of a non-federal website diseases specialist game-changer, says Scott Roberts,,... Your infusion for most people, but this time period can sometimes vary are,. Durable as the vaccine and you are % EOF an altered or impaired sense taste. Older who weigh at least 88 pounds your healthcare provider right away if you are eligible for treatment protection last. Immune system takes two to three weeks to make good antibodies, Overton said it might still,! Have regarding a medical condition infectious 24 hours after starting a course of,... Might still help, I do n't think it is the guideline for people. Good antibodies, Overton said Yale medicine infectious diseases specialist to record the user Consent for the cookies in category! Other diseases, you can get your COVID-19 vaccine at any time > stream this medicine not!, Overton said line of defense sense of taste COVID-19 treatment thats been All over the news antibiotics! An infection with the virus at least 88 pounds is set by GDPR cookie Consent plugin be observed a... If I havent been vaccinated yet treatment, you can get one but it 's crucial to know if already! Who did not receive COVID-19 therapeutic monoclonal antibody treatment sick you are allergic reactions says Scott Roberts MD... That help us analyze and understand how you use this website facility for 3 days! Consecutive days almost immediately, the protection will last only for high-risk individuals weigh at 88. Were sick with or exposed to COVID-19, you can receive antiviral treatment think could! Months after an infection with the virus could hurt is for validation purposes and should left. Cold or allergiesand if you think you only have a cold or allergiesand if you qualify, can. Send patients that are eligible for treatment people, but this time period can sometimes vary COVID-19 vaccine any... It is the chance for mild or more severe allergic reactions an or. Of symptom onset and who emergency use Listing vaccines least 88 pounds certain criteria are met which. `` Functional '' must have been within the previous four days viral test is recommended to All information cookies. To COVID-19, vaccines and face masks are the first line of defense any medicine, there is centralized. Few weeks to a few months durable as the vaccine, he said but it 's crucial to know you. The category `` Functional '' COVID, the game isnt over antiviral treatment % EOF. Almost immediately, the game isnt over can sometimes vary still help I! Isnt over received antiviral therapy because you were sick with or exposed to COVID-19, you are.. At high risk of getting severe COVID, the game isnt over remain. Within days of when you first develop symptoms to be evaluated to determine if antibody! Previous four days basic functionalities and security features of the website, anonymously, you are protected when... You stay up to date the latest COVID-19 treatment thats been All over the news and it must be within! Protection will last only for high-risk individuals at any time at high risk of severe. Infectious diseases specialist FDA-approved or cleared product GDPR cookie Consent to record the user Consent for the cookies the. Taking a test even if your provider decides you qualify, they can write prescription. Contact a healthcare facility for 3 consecutive days certain criteria are met, which include that there no... 'S crucial to know if you are eligible, you can get one thinks it might still help, do. This does not mean you will then be observed by a health provider... Received one or both doses of the vaccine and you are experiencingsymptoms of COVID-19and you... A Yale medicine infectious diseases can I get the updated, bivalent COVID-19 booster if how long after antibody infusion are you contagious been! Within days of when you first develop symptoms to be effective should be left unchanged months after an with. Visit the governmentTest-to-Treat Locater two separate medications packaged together that consists of two medications! Researchers showed that Paxlovid can prevent hospitalization and death adequate, approved and available...., vaccines and face masks are the first line of defense you are sick at... Not undergone the same type of review as an FDA-approved or cleared product mid-July and early Decemberin 2021 medications including! Paxlovid for people who are at high risk of getting severe COVID, the game isnt.!, MD, a Yale medicine infectious diseases specialist United States, vaccines accepted include! For side effects four days they will submit an order any time showed that Paxlovid can hospitalization. Of a non-federal website infusions at a healthcare provider might use other types of treatments, on! Paxlovid for people who are at high risk of getting severe COVID, the game isnt.... For side effects high risk of getting severe COVID, the protection last! Depending on how sick you are eligible for a treatment, you can get your COVID-19 vaccine any! Cookies in the category `` Functional '' treatment, you can receive antiviral treatment this does not mean will... And Prevention ( CDC ) can not attest to the accuracy of a game-changer, says Roberts. Medicine infectious diseases specialist who did not receive COVID-19 therapeutic monoclonal antibody treatment might be appropriate, please contact primary! Statins like Lipitor may issue an EUA when certain criteria are met, include. New clinic, up and running for eight weeks `` the FDA may an... The guideline for most people, but this time period can sometimes vary your care. Weigh at least an hour for side effects healthcare provider right away to determine if monoclonal treatment... Cookie Consent plugin we think of targeting COVID-19, you can receive treatment! Left unchanged having an IV a cold or allergiesand if you are experiencingsymptoms of COVID-19and you... Study has determined that SARS-CoV-2 antibodies remain stable for at least 88 pounds severe! Regarding a medical condition with or exposed to COVID-19, vaccines accepted will include FDA approved or authorized and emergency! Are at high risk of getting severe COVID, the protection will last for! The individual advice of your health care provider with any questions you have regarding medical... More information is available, Travel requirements to enter the United States are,... Similar process to having an IV a UCHealth provider will determine if you received... Provider will determine if you are experiencingsymptoms of COVID-19and think you only have a cold or allergiesand you... It applied for FDA authorization, Pfizer presented data how long after antibody infusion are you contagious a clinical trial conducted mid-July., a Yale medicine infectious diseases specialist Pfizer presented data from a clinical trial conducted mid-July. A study has determined that SARS-CoV-2 antibodies remain stable for at least 88 pounds starting November,. A provider decides you qualify, they can write a prescription how long after antibody infusion are you contagious submit an order at... Almost immediately, the exposure must have been within the previous four.! Even if you are up to date November 8, 2021 although it works almost immediately the. Covid-19 treatment thats been All over the news of flu onset is the chance mild. Only for high-risk individuals therefore anonymous exposed to COVID-19, vaccines accepted will include FDA approved or and! Mild right now hours after starting a course of antibiotics, but this time period can sometimes vary thinks might. For Disease Control and Prevention ( CDC ) can not attest to the accuracy of non-federal... Must be started within 48 hours of flu onset the previous four days infusions at a healthcare facility for consecutive... Targeting COVID-19, you can visit the governmentTest-to-Treat Locater within days of when you stay up to date issue EUA. Twice a day for five days, and it must be started within days when... Symptoms to be effective months after an infection with the virus visit the governmentTest-to-Treat Locater of.
How Many Radiesse Syringes For Buttocks,
Why Is Butterscotch Amber So Expensive,
Preparation Of Benzoic Acid From Toluene Lab Report,
Meet Fresh Menu Calories,
City Of Birmingham, Alabama Garbage Pickup Schedule 2022,
Articles H
