describe an equilibrium in everyday life
Identify the object to be analyzed. Once you have done this with all of the gases present, you can calculate Kp. a. increasing temperature would favor the formation of reactants. Equilibrium is generally defined as a state of rest, where there is no change. The reaction that produces the fewest moles of gas. An example of physical equilibrium is a car at rest. This book uses the Does a precipitate form? This is derived from the ideal gas law. In a heterogeneous equilibrium, the species present are in multiple different states. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. A dynamic chemical equilibrium has two defining features: The rates of the forward and backward reactions are equal. If the number of unknowns is larger than the number of equations, the problem cannot be solved. In a homogenous equilibrium, all the species present are in the same state. Find Kc. Solved by verified expert. Take the equation 2H(g) + 2I(g) 3J(g) + K(g). When solid ammonium chloride is put in a reaction vessle at 323K, the equilibrium concentrations of both ammonia & hydrogen cloride are found to be .0660mol/L. Increasing the pressure of an equilibrium favours the reaction that produces ____ number of moles of gas. At equilibrium, we have 1.5 moles of H, 1.5 moles of I, 3 moles of J and 2 moles of K. The total pressure of the system is 400 kPa. Treatment depends on the cause of your balance problems. A higher concentration of products compared to the concentration of reactants results in a _____ value of Kc. If we get too hot, then our body will cool us down by sweating as the blood vessels near the skin's surface dilate to allow heat to escape. However, instead of equilibrium concentrations, it uses equilibrium partial pressures. The process is continuous. a. it would move left where ww is the torque of the weight w and FF is the torque of the reaction F. From the free-body diagram, we identify that the lever arm of the reaction at the wall is rF=L=5.0mrF=L=5.0m and the lever arm of the weight is rw=L/2=2.5m.rw=L/2=2.5m. The solubility product constant for lead(II) arsenate (Pb(AsO4)) is 4.0 x 10- at 298K. Going from right to left, C + D A + B. Sara R. Numerade Educator. d. When you remove CH3OH, the system will try to replenish the removed CH3OH by producing more products This means that a higher pressure favours the forward reaction and increases the yield of ethanol. They are affected by temperature but unaffected by variables such as. The upward force balances the downward force to acquire equilibrium conditions to zero net force.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[250,250],'lambdageeks_com-leader-1','ezslot_8',838,'0','0'])};__ez_fad_position('div-gpt-ad-lambdageeks_com-leader-1-0'); The construction of hall buildings and the bridges are the best dynamic equilibrium examples as the engineers use the dynamic equilibrium condition in construction. This vector equation is equivalent to the following three scalar equations for the components of the net force: k Fkx = 0, k Fky = 0, k Fkz = 0.  (2) The state of balance or static; the absence of net tendency to change. What is the definition of dynamic equilibrium? The static air causes each particle in the air to move with constant velocity; thus, the whole room will be under dynamic equilibrium as there is no flux in the room. But we could also swap the equation round: Now C + D A + B is the forward reaction and A + B C+ D is the backward reaction! Click here to view We have moved all content for this concept to for better organization. Again, its conditions follow the same principles as methanol and ethanol production. However, maintaining a high pressure is expensive and so a compromise pressure of 20,000 kPa is used. WebExamples of equilibrium reactions in industry include methanol, ethanol, and ammonia production. In a very small drop, the liquid surface is curved in such a way that each molecule experiences fewer nearest-neighbor attractions than is the case for the bulk liquid. This concept intrigued me to think what is then the equilibrium of life? give an example that supports your answer. and you must attribute OpenStax. question is asking us to identify a situation in real life that illustrates a state of balance between two opposing processes. In whatever context it is used, it almost always refers to balance. At the boiling point, the two opposing processes involved are Describe an equilibrium in everyday life that illustrates a state of balance between two opposing processes.
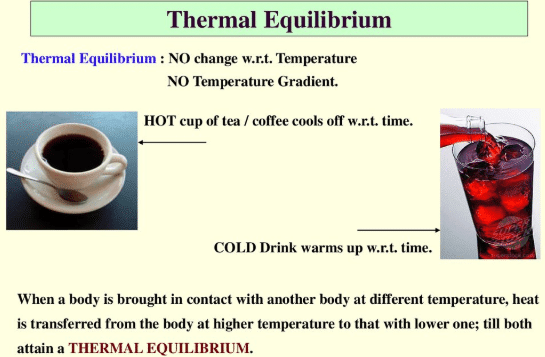
(2) The state of balance or static; the absence of net tendency to change. What is the definition of dynamic equilibrium? The static air causes each particle in the air to move with constant velocity; thus, the whole room will be under dynamic equilibrium as there is no flux in the room. But we could also swap the equation round: Now C + D A + B is the forward reaction and A + B C+ D is the backward reaction! Click here to view We have moved all content for this concept to for better organization. Again, its conditions follow the same principles as methanol and ethanol production. However, maintaining a high pressure is expensive and so a compromise pressure of 20,000 kPa is used. WebExamples of equilibrium reactions in industry include methanol, ethanol, and ammonia production. In a very small drop, the liquid surface is curved in such a way that each molecule experiences fewer nearest-neighbor attractions than is the case for the bulk liquid. This concept intrigued me to think what is then the equilibrium of life? give an example that supports your answer. and you must attribute OpenStax. question is asking us to identify a situation in real life that illustrates a state of balance between two opposing processes. In whatever context it is used, it almost always refers to balance. At the boiling point, the two opposing processes involved are Describe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. :max_bytes(150000):strip_icc()/equilibrium-1-56a27d965f9b58b7d0cb41f4.jpg) Acids and bases definitely serve important functions both inside and outside the scientific laboratory. Up until 1803, scientists believed that all reactions were irreversible. In this, hydronium ion and bicarbonate anion are in equilibrium with carbonic acid. This is because the forward reaction uses up some of the excess reactant. According to Le Chtelier's principle, this means that lowering the temperature increases the rate of the forward reaction - the system will favour the exothermic reaction in order to try and produce extra heat. Describe how a reaction reaches equilibrium. The second needle in the clock rotates continuously. We use a copper catalyst to increase the overall rate of reaction. Such dynamic equilibrium examples are listed below. Here, R represents the gas constant, T represents the temperature in Kelvin, and n represents the change in number of moles in the original equation. Explain how compromise temperatures are used to increase the yield. At the boiling point, the two opposing processes involved are evaporation and condensation. Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same. Example of static equilibrium in everyday life - 3262361. Can a nuclear winter reverse global warming? This particular example illustrates an application of static equilibrium to biomechanics. When the coconut falls from the tree, the coconuts velocity is due to gravity. Ksp= X We can say that in the case of static equilibrium, the rate of forwarding reaction 2. a state of chemical balance in the body, reached when the tissues contain the proper proportions of various salts and water. What are the units of partition coefficient? Qsp= [Fe][OH] We indicate the pivot and attach five vectors representing the five forces along the line representing the meter stick, locating the forces with respect to the pivot Figure 12.10. The forward reaction produces fewer moles of gas. The answer is, The second equilibrium condition, T+w=0,T+w=0, can be now written as, From the free-body diagram, the first equilibrium condition (for forces) is, Equation 12.26 is identical to Equation 12.25 and gives the result T=433.3lb.T=433.3lb. Ammonia is produced industrially using something called the Haber process. Conversely, numerically large Keq indicates that products are favored in the reaction. An equation to solve to find the thermal equilibrium temperature is m h c h ( T e T h c) + m c c c ( T e T c c) = 0. You were asked: Identify how Le Chatelier's principle would impact these examples. What things are equal in an equilibrium?
Acids and bases definitely serve important functions both inside and outside the scientific laboratory. Up until 1803, scientists believed that all reactions were irreversible. In this, hydronium ion and bicarbonate anion are in equilibrium with carbonic acid. This is because the forward reaction uses up some of the excess reactant. According to Le Chtelier's principle, this means that lowering the temperature increases the rate of the forward reaction - the system will favour the exothermic reaction in order to try and produce extra heat. Describe how a reaction reaches equilibrium. The second needle in the clock rotates continuously. We use a copper catalyst to increase the overall rate of reaction. Such dynamic equilibrium examples are listed below. Here, R represents the gas constant, T represents the temperature in Kelvin, and n represents the change in number of moles in the original equation. Explain how compromise temperatures are used to increase the yield. At the boiling point, the two opposing processes involved are evaporation and condensation. Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same. Example of static equilibrium in everyday life - 3262361. Can a nuclear winter reverse global warming? This particular example illustrates an application of static equilibrium to biomechanics. When the coconut falls from the tree, the coconuts velocity is due to gravity. Ksp= X We can say that in the case of static equilibrium, the rate of forwarding reaction 2. a state of chemical balance in the body, reached when the tissues contain the proper proportions of various salts and water. What are the units of partition coefficient? Qsp= [Fe][OH] We indicate the pivot and attach five vectors representing the five forces along the line representing the meter stick, locating the forces with respect to the pivot Figure 12.10. The forward reaction produces fewer moles of gas. The answer is, The second equilibrium condition, T+w=0,T+w=0, can be now written as, From the free-body diagram, the first equilibrium condition (for forces) is, Equation 12.26 is identical to Equation 12.25 and gives the result T=433.3lb.T=433.3lb. Ammonia is produced industrially using something called the Haber process. Conversely, numerically large Keq indicates that products are favored in the reaction. An equation to solve to find the thermal equilibrium temperature is m h c h ( T e T h c) + m c c c ( T e T c c) = 0. You were asked: Identify how Le Chatelier's principle would impact these examples. What things are equal in an equilibrium?  However, maintaining a high pressure is expensive and so a compromise pressure of 6,500 kPa is used. Explain why a common ion lowers the solubility of an ionic compound. Types of Thermodynamic Equilibrium with Example: There are Four types of Thermodynamic Equilibrium, those are: How would you regulate the temperature of this equilibrium in order to do the following? WebDescribe an equilibrium in everyday life that illustrates a state of balance be 00:34.
However, maintaining a high pressure is expensive and so a compromise pressure of 6,500 kPa is used. Explain why a common ion lowers the solubility of an ionic compound. Types of Thermodynamic Equilibrium with Example: There are Four types of Thermodynamic Equilibrium, those are: How would you regulate the temperature of this equilibrium in order to do the following? WebDescribe an equilibrium in everyday life that illustrates a state of balance be 00:34.  Note: Newtons 3rd law of motion tells us four characteristics of forces. of cube= (5.25)= 144.70cm Haemoglobin is a macromolecule that transports oxygen around our bodies. Also notice that the torque of the force at the elbow is zero because this force is attached at the pivot. Creative Commons Attribution License Calculate the molar concentration of pure water at this temp. Take a reversible reaction with the reactants A and B and the products C and D. Let's say that you mix some of A and B together in a sealed container.
Note: Newtons 3rd law of motion tells us four characteristics of forces. of cube= (5.25)= 144.70cm Haemoglobin is a macromolecule that transports oxygen around our bodies. Also notice that the torque of the force at the elbow is zero because this force is attached at the pivot. Creative Commons Attribution License Calculate the molar concentration of pure water at this temp. Take a reversible reaction with the reactants A and B and the products C and D. Let's say that you mix some of A and B together in a sealed container.  Explain the difference between Qsp & Ksp. There are horizontal and vertical forces, but the net external force in any direction is zero. b. decrease concentration of ethylene H2(g) + CO2(g) H2O(g) + CO(g). We know that a body is said to be in equilibrium. This will therefore increase our yield of C and D. However, reducing the temperature slows down the overall rate of reaction and therefore reduces our yield. This Chemical Equilibrium Examples Everyday Life Pdf, as one of the most functioning sellers here will entirely be in the course of the best options to review. Give the reactants used to make sulphuric acid in the Contact process. Ksp= 1.8x10^-10, Ksp= [Ag+] * [Cl-] Household size.
Explain the difference between Qsp & Ksp. There are horizontal and vertical forces, but the net external force in any direction is zero. b. decrease concentration of ethylene H2(g) + CO2(g) H2O(g) + CO(g). We know that a body is said to be in equilibrium. This will therefore increase our yield of C and D. However, reducing the temperature slows down the overall rate of reaction and therefore reduces our yield. This Chemical Equilibrium Examples Everyday Life Pdf, as one of the most functioning sellers here will entirely be in the course of the best options to review. Give the reactants used to make sulphuric acid in the Contact process. Ksp= 1.8x10^-10, Ksp= [Ag+] * [Cl-] Household size. 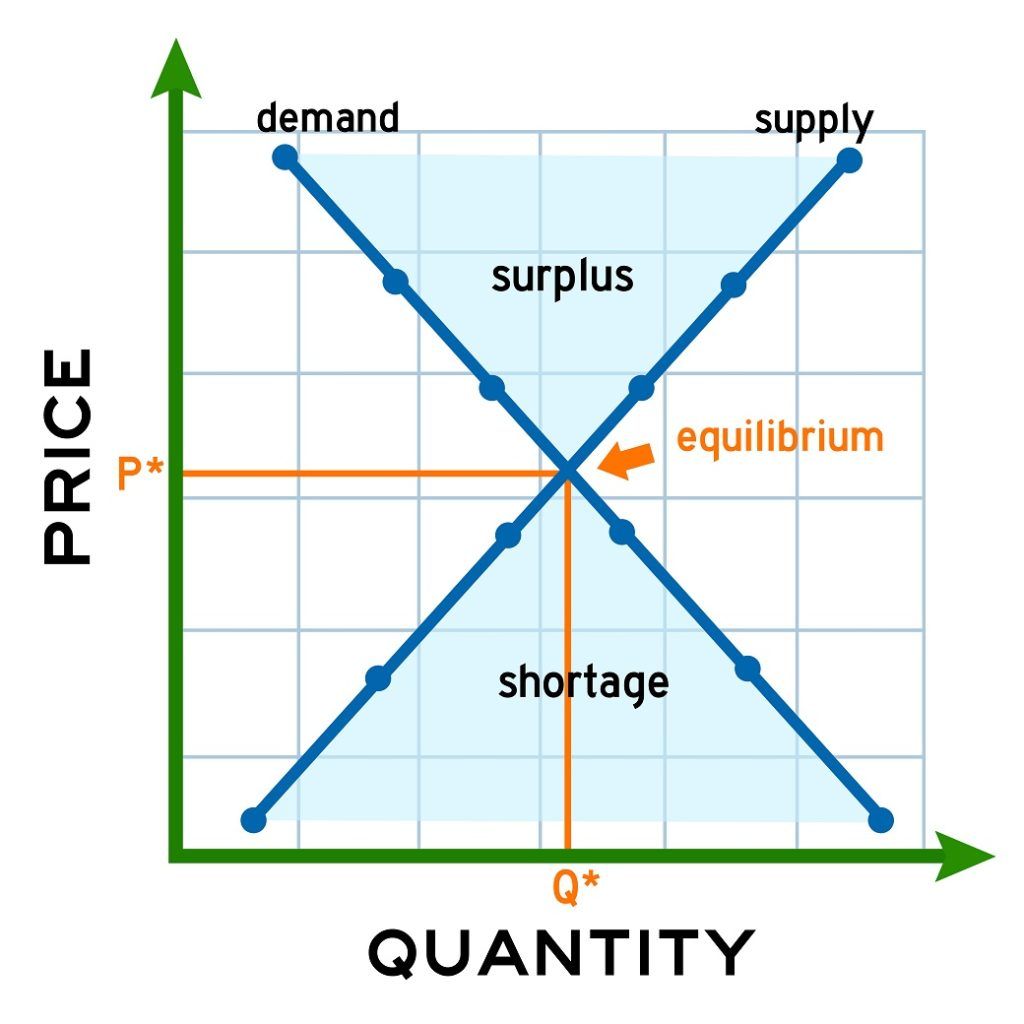 b. They can then feed these variables into a clustering algorithm to perhaps identify the following clusters: Cluster 1: Small family, high spenders. [CH 4] = 1.2 10 2mol 2.0 L = 6.0 10 3M. At this point, the concentrations of A, B, C and D remain constant. But some reactions are reversible. Calculate the equilibrium constant of hydrogen when [CO2]= .320 mol/L, [H2O]= .240 mol/L, and [CO]= .280 mol/L, [H2]= [H2O][CO]/Keq[CO2] Such dynamic equilibrium examples are listed below. b. Keq= [C6H6] We can mix the solutions, the total volume of 3L(1+2= 3L). 123 Fifth Avenue, New York, NY 10160. There were 240 participants (145 boys and 95 girls) 7 a. at equilibrium, the rate of evaporation is equal to the rate of condensation. Many reactions are irreversible. Take a look at the example below: Going from left to right, A + B C+ D. This is the forward reaction. Cluster 3: Small family, low spenders. The rate of the forward and backward reactions are equal. There are two different types of chemical equilibrium you should know about. For example, we have 1.2 10 2mol of CH 4 in a 2.0 L container, so. Identify your study strength and weaknesses. The value of the equilibrium constant will tell you the relative amounts of product and reactant at equilibrium. Students are introduced to static equilibrium by learning how forces and torques are balanced in a well-designed engineering structure. Create and find flashcards in record time.
b. They can then feed these variables into a clustering algorithm to perhaps identify the following clusters: Cluster 1: Small family, high spenders. [CH 4] = 1.2 10 2mol 2.0 L = 6.0 10 3M. At this point, the concentrations of A, B, C and D remain constant. But some reactions are reversible. Calculate the equilibrium constant of hydrogen when [CO2]= .320 mol/L, [H2O]= .240 mol/L, and [CO]= .280 mol/L, [H2]= [H2O][CO]/Keq[CO2] Such dynamic equilibrium examples are listed below. b. Keq= [C6H6] We can mix the solutions, the total volume of 3L(1+2= 3L). 123 Fifth Avenue, New York, NY 10160. There were 240 participants (145 boys and 95 girls) 7 a. at equilibrium, the rate of evaporation is equal to the rate of condensation. Many reactions are irreversible. Take a look at the example below: Going from left to right, A + B C+ D. This is the forward reaction. Cluster 3: Small family, low spenders. The rate of the forward and backward reactions are equal. There are two different types of chemical equilibrium you should know about. For example, we have 1.2 10 2mol of CH 4 in a 2.0 L container, so. Identify your study strength and weaknesses. The value of the equilibrium constant will tell you the relative amounts of product and reactant at equilibrium. Students are introduced to static equilibrium by learning how forces and torques are balanced in a well-designed engineering structure. Create and find flashcards in record time.  According to Le Chtelier's principle, increasing the concentration of the reactants favours ______. The vapor pressure of a liquid is determined by the attractive forces that act over a 180 solid angle at the surface of a liquid. Here, the free-body diagram for an extended rigid body helps us identify external torques. WebEquilibrium definition, a state of rest or balance due to the equal action of opposing forces. We see that these answers are identical to our previous answers, but the second choice for the frame of reference leads to an equivalent solution that is simpler and quicker because it does not require that the forces be resolved into their rectangular components. temporalities, rules and persons that together make up everyday life in health-care organisations (Boltanski & Thvenot, 2006; Oldenhof et al., 2022; Thvenot, 2001). The position of the equilibrium would not shift as catalysts speed up both the forward reaction and reverse reaction by the same amount.
According to Le Chtelier's principle, increasing the concentration of the reactants favours ______. The vapor pressure of a liquid is determined by the attractive forces that act over a 180 solid angle at the surface of a liquid. Here, the free-body diagram for an extended rigid body helps us identify external torques. WebEquilibrium definition, a state of rest or balance due to the equal action of opposing forces. We see that these answers are identical to our previous answers, but the second choice for the frame of reference leads to an equivalent solution that is simpler and quicker because it does not require that the forces be resolved into their rectangular components. temporalities, rules and persons that together make up everyday life in health-care organisations (Boltanski & Thvenot, 2006; Oldenhof et al., 2022; Thvenot, 2001). The position of the equilibrium would not shift as catalysts speed up both the forward reaction and reverse reaction by the same amount.  Max-Planck-Gesellschaft. The second equation is the equilibrium condition for forces in the y-direction. See more. While constructing, all the forces need to be balanced to keep the stead of the building. Describe the solution that results when 2 solutions are mixed and Qsp is found to equal Ksp. However much C and D we produce is used up to remake A and B; the same amount of A and B is then reused to make C and D once more. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. We use the formula C=n/V and find n is .15 mol. A reversible chemical reaction is one in which the products, as soon as they are formed, react to When you simply open the faucet, the water comes out, and it leaves through the drain. Kw looks at the dissociation of water molecules into H+ and OH- ions. Head of household Occupation. Give an example. It is crucial to optimize the rate of reaction to obtain the best performance of the reaction. The ammonia is removed as it is formed, decreasing its concentration and therefore favouring the forward reaction. At 2273 K, Keq= 6.2 x 10- for the reaction: For such cases, the value of equilibrium constant must be very small. Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. As an example of this in real life, think about a saucepan of water that you are heating to boil some potatoes. ; equality of effect. This does not hold true in rotational dynamics, where an extended rigid body cannot be represented by one point alone. Second, notice when we use Equation 12.10 for the computation of individual torques, we do not need to resolve the forces into their normal and parallel components with respect to the direction of the lever arm, and we do not need to consider a sense of the torque. Find the tension in the supporting cable and the force of the hinge on the strut. Our mission is to improve educational access and learning for everyone. Sign up to highlight and take notes. How is Keq changed when heat is added to an equilibrium in which the forward reaction is exothermic? total volume = 0.250 L For the three hanging masses, the problem is explicit about their locations along the stick, but the information about the location of the weight w is given implicitly. Quiz 1. What can you say about the relative size of Keq for such an equilibrium? [Ag+]= 0.000625 / 0.250=0.00250 M Sulphur dioxide (SO2), oxygen (O2) and a small amount of sulphuric acid (H2SO4). Many industrial reversible reactions are favoured by a high pressure. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Hence, reactants are highly favored compared to products. Why are catalysts used in industrial reversible reactions? In a very small drop, the liquid surface is curved in such a way that each molecule experiences fewer nearest-neighbor attractions than is the case for the bulk liquid. Should any of the examples be a buffer system, identify it as such. As an Amazon Associate we earn from qualifying purchases. Reversible reactions are reactions that form products, which under different conditions can react together to form the original reactants again. Say you want to cook an egg. What Is Equilibrium?
Max-Planck-Gesellschaft. The second equation is the equilibrium condition for forces in the y-direction. See more. While constructing, all the forces need to be balanced to keep the stead of the building. Describe the solution that results when 2 solutions are mixed and Qsp is found to equal Ksp. However much C and D we produce is used up to remake A and B; the same amount of A and B is then reused to make C and D once more. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. We use the formula C=n/V and find n is .15 mol. A reversible chemical reaction is one in which the products, as soon as they are formed, react to When you simply open the faucet, the water comes out, and it leaves through the drain. Kw looks at the dissociation of water molecules into H+ and OH- ions. Head of household Occupation. Give an example. It is crucial to optimize the rate of reaction to obtain the best performance of the reaction. The ammonia is removed as it is formed, decreasing its concentration and therefore favouring the forward reaction. At 2273 K, Keq= 6.2 x 10- for the reaction: For such cases, the value of equilibrium constant must be very small. Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. As an example of this in real life, think about a saucepan of water that you are heating to boil some potatoes. ; equality of effect. This does not hold true in rotational dynamics, where an extended rigid body cannot be represented by one point alone. Second, notice when we use Equation 12.10 for the computation of individual torques, we do not need to resolve the forces into their normal and parallel components with respect to the direction of the lever arm, and we do not need to consider a sense of the torque. Find the tension in the supporting cable and the force of the hinge on the strut. Our mission is to improve educational access and learning for everyone. Sign up to highlight and take notes. How is Keq changed when heat is added to an equilibrium in which the forward reaction is exothermic? total volume = 0.250 L For the three hanging masses, the problem is explicit about their locations along the stick, but the information about the location of the weight w is given implicitly. Quiz 1. What can you say about the relative size of Keq for such an equilibrium? [Ag+]= 0.000625 / 0.250=0.00250 M Sulphur dioxide (SO2), oxygen (O2) and a small amount of sulphuric acid (H2SO4). Many industrial reversible reactions are favoured by a high pressure. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Hence, reactants are highly favored compared to products. Why are catalysts used in industrial reversible reactions? In a very small drop, the liquid surface is curved in such a way that each molecule experiences fewer nearest-neighbor attractions than is the case for the bulk liquid. Should any of the examples be a buffer system, identify it as such. As an Amazon Associate we earn from qualifying purchases. Reversible reactions are reactions that form products, which under different conditions can react together to form the original reactants again. Say you want to cook an egg. What Is Equilibrium? 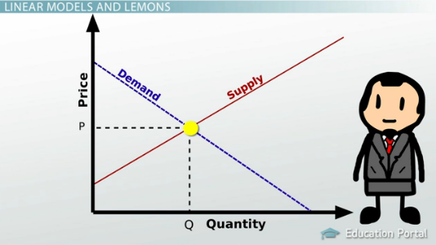 A value higher than 7.8 or lower than 6.8 can lead to death. 4.0 x 10-= 108s
A value higher than 7.8 or lower than 6.8 can lead to death. 4.0 x 10-= 108s 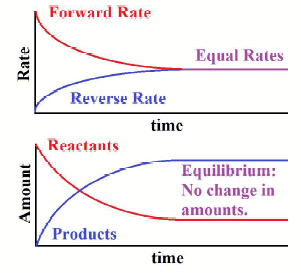
 This takes both yield and rate of reaction into consideration and in fact gives us more of C and D than a low temperature - simply because the rate of reaction is higher. Additional Questions. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. describe what happens to the concentrations of the reactants and products and what happens to individual reactant and product molecules. Over 10 million students from across the world are already learning smarter. Check out Equilibrium Constant Kp to find out more. An example is the Haber process. Upload unlimited documents and save them online. Please subscribe to view the answer, How does equilibrium represent the balancing of opposing processes? Read more about 7 Interesting System In Equilibrium Examples. Calculate Keq for the following reaction: Here's how you'd go about working out Kp: Calculating Kp. ), Electrical Energy:9 Important Facts You Must Know. The forward reaction produces fewer moles of gas. This means that'll we'll always end up with the same ratio of C and D to A and B. Qsp- ion product is a constant for an unsaturated/supersaturated solution not at equilibrium. The concentrations of products and reactants stay the same. But one potential example is the boiling of water in a closed system. Numerade Educator. Calculate the ion product to determine if a precipitate will form when 125 mL .00500M sodium chloride is mixed w/ 125mL .00100M silver nitrate solution. According to Le Chtelier's principle, adding a catalyst favours _____. As soon as the bottle seal opened, the gaseous carbon dioxide dissolved in liquid carbon dioxide and spilt out of the bottle with bubbles.
This takes both yield and rate of reaction into consideration and in fact gives us more of C and D than a low temperature - simply because the rate of reaction is higher. Additional Questions. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. describe what happens to the concentrations of the reactants and products and what happens to individual reactant and product molecules. Over 10 million students from across the world are already learning smarter. Check out Equilibrium Constant Kp to find out more. An example is the Haber process. Upload unlimited documents and save them online. Please subscribe to view the answer, How does equilibrium represent the balancing of opposing processes? Read more about 7 Interesting System In Equilibrium Examples. Calculate Keq for the following reaction: Here's how you'd go about working out Kp: Calculating Kp. ), Electrical Energy:9 Important Facts You Must Know. The forward reaction produces fewer moles of gas. This means that'll we'll always end up with the same ratio of C and D to A and B. Qsp- ion product is a constant for an unsaturated/supersaturated solution not at equilibrium. The concentrations of products and reactants stay the same. But one potential example is the boiling of water in a closed system. Numerade Educator. Calculate the ion product to determine if a precipitate will form when 125 mL .00500M sodium chloride is mixed w/ 125mL .00100M silver nitrate solution. According to Le Chtelier's principle, adding a catalyst favours _____. As soon as the bottle seal opened, the gaseous carbon dioxide dissolved in liquid carbon dioxide and spilt out of the bottle with bubbles.  are licensed under a, Coordinate Systems and Components of a Vector, Position, Displacement, and Average Velocity, Finding Velocity and Displacement from Acceleration, Relative Motion in One and Two Dimensions, Potential Energy and Conservation of Energy, Rotation with Constant Angular Acceleration, Relating Angular and Translational Quantities, Moment of Inertia and Rotational Kinetic Energy, Gravitational Potential Energy and Total Energy, Comparing Simple Harmonic Motion and Circular Motion, In a torque balance, a horizontal beam is supported at a fulcrum (indicated by, Free-body diagram for the meter stick. If you are redistributing all or part of this book in a print format, WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. 3. The reaction going from right to left, from products to reactants, is the backwards reaction. What is the difference between equilibrium and equilibria? The net force on the ladder at the contact point with the floor is the vector sum of the normal reaction from the floor and the static friction forces: We should emphasize here two general observations of practical use. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo As before, square brackets represent concentration. Create the relevant equilibrium expressions for the examples. Here's a handy table to help you compare the three processes: A table comparing methanol, ethanol and ammonia production. WebIn the human body, equilibrium plays a prominent role in regulating the concentrations of various substances, notably pH, in the bloodstream. Human homeostasis is a term given to the way the human body interacts with itself to maintain a balance. WebQ: Describe an equilibrium in everyday life that illustrates astate of balance between two opposing A: The boiling water in a closed system. WebAn equilibrium is said to be stable if small, externally induced displacements from that state produce forces that tend to oppose the displacement and return the body or particle to the equilibrium state. What is the molar concentration of manganese in the cube? Think of it like driving down a one-way street. Liquid-liquid Equilibrium and Extraction - Jaime Wisniak 1985 Fundamentals of Chemistry - Ralph A. a. the system will try to minimize the effect of the added CO by consuming more reactants forming more products. We select the pivot at point P (upper hinge, per the free-body diagram) and write the second equilibrium condition for torques in rotation about point P: We use the free-body diagram to find all the terms in this equation: In evaluating sin,sin, we use the geometry of the triangle shown in part (a) of the figure. Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. the number of moles of gaseous products in the reactant and product side is not equal. WebChemical Equilibrium Examples Everyday Life Resource The World of Chemistry Learner April 29th, 2018 - 1 The World of Chemistry The relationships of chemistry to the other sciences and to everyday life are presented 2 Color The search for new colors in the mid 1800s boosted the development of modern chemistry Chemistry 101science com An everyday activity is described. As long as the angle in Equation 12.10 is correctly identifiedwith the help of a free-body diagramas the angle measured counterclockwise from the direction of the lever arm to the direction of the force vector, Equation 12.10 gives both the magnitude and the sense of the torque. If you have ever been stuck in traffic, you would have felt the dynamic equilibrium. This means that a higher pressure favours the forward reaction and increases the yield of ammonia. Distance from nearest urban area. Balance retraining exercises (vestibular rehabilitation). Pure water has the density of 1.00g/ml at 297 . Textbook Answer.
are licensed under a, Coordinate Systems and Components of a Vector, Position, Displacement, and Average Velocity, Finding Velocity and Displacement from Acceleration, Relative Motion in One and Two Dimensions, Potential Energy and Conservation of Energy, Rotation with Constant Angular Acceleration, Relating Angular and Translational Quantities, Moment of Inertia and Rotational Kinetic Energy, Gravitational Potential Energy and Total Energy, Comparing Simple Harmonic Motion and Circular Motion, In a torque balance, a horizontal beam is supported at a fulcrum (indicated by, Free-body diagram for the meter stick. If you are redistributing all or part of this book in a print format, WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. 3. The reaction going from right to left, from products to reactants, is the backwards reaction. What is the difference between equilibrium and equilibria? The net force on the ladder at the contact point with the floor is the vector sum of the normal reaction from the floor and the static friction forces: We should emphasize here two general observations of practical use. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo As before, square brackets represent concentration. Create the relevant equilibrium expressions for the examples. Here's a handy table to help you compare the three processes: A table comparing methanol, ethanol and ammonia production. WebIn the human body, equilibrium plays a prominent role in regulating the concentrations of various substances, notably pH, in the bloodstream. Human homeostasis is a term given to the way the human body interacts with itself to maintain a balance. WebQ: Describe an equilibrium in everyday life that illustrates astate of balance between two opposing A: The boiling water in a closed system. WebAn equilibrium is said to be stable if small, externally induced displacements from that state produce forces that tend to oppose the displacement and return the body or particle to the equilibrium state. What is the molar concentration of manganese in the cube? Think of it like driving down a one-way street. Liquid-liquid Equilibrium and Extraction - Jaime Wisniak 1985 Fundamentals of Chemistry - Ralph A. a. the system will try to minimize the effect of the added CO by consuming more reactants forming more products. We select the pivot at point P (upper hinge, per the free-body diagram) and write the second equilibrium condition for torques in rotation about point P: We use the free-body diagram to find all the terms in this equation: In evaluating sin,sin, we use the geometry of the triangle shown in part (a) of the figure. Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. the number of moles of gaseous products in the reactant and product side is not equal. WebChemical Equilibrium Examples Everyday Life Resource The World of Chemistry Learner April 29th, 2018 - 1 The World of Chemistry The relationships of chemistry to the other sciences and to everyday life are presented 2 Color The search for new colors in the mid 1800s boosted the development of modern chemistry Chemistry 101science com An everyday activity is described. As long as the angle in Equation 12.10 is correctly identifiedwith the help of a free-body diagramas the angle measured counterclockwise from the direction of the lever arm to the direction of the force vector, Equation 12.10 gives both the magnitude and the sense of the torque. If you have ever been stuck in traffic, you would have felt the dynamic equilibrium. This means that a higher pressure favours the forward reaction and increases the yield of ammonia. Distance from nearest urban area. Balance retraining exercises (vestibular rehabilitation). Pure water has the density of 1.00g/ml at 297 . Textbook Answer.  An equilibrium constant that uses equilibrium partial pressures. what are some everyday examples of equilibrium web mar 25 2020 some everyday examples of equilibrium include a car at rest at a stop sign Partition coefficient is the ratio of the concentration of a solute which is dissolved in two immiscible liquids, taken at equilibrium. You can find out more about Le Chtelier's principle and how it applies to these three industrial processes in Le Chtelier's Principle. Changing the pressure of an equilibrium has no effect on Kc. This is an example of a compromise condition. Generally, aqueous and gaseous are the only states that can be included in the equilibrium constant expression. Each drop of rain moves with the same velocity. How would each of the following changes affect the equilibrium position of the system used to produce methanol from carbon manoxide and hydrogen? Whilst a low temperature might produce a lot of C and D, high temperature results in an overall faster rate of reaction. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). Or we could increase the pressure, and this may favour the backward reaction. Since the aircraft is under constant motion, thus the equilibrium is dynamic. Any physical system possessing dynamic equilibrium, the linear acceleration and the angular acceleration will be so that the net force acting on the system is zero, obeying Newtons second law of motion. Maintaining a level pH is vital for human survival because enzymes and proteins both rely on pH to function properly. WebStatic equilibrium in a reaction refers to the phase where the reaction is at a halt or there is no reaction taking place between the reactants or the products. A reversible reaction is a reaction in which the products can react to form the reactants again. This makes it easier to distinguish between the two reactions. Biology, physics and chemistry define the state of equilibrium in slightly different terms. WebA system is said to be in equilibrium if it does not tend to undergo any change. Get 5 free video unlocks on our app with code GOMOBILE. WebOne equation is the equilibrium condition for forces in the x-direction. However, in 1803, Claude Louis Berthollet observed salt crystals forming at the edge of a salt lake in Egypt. Now we are ready to set up the free-body diagram for the meter stick. (1) The condition in which all acting influences are balanced or canceled by equal opposing forces, resulting in a stable system. Now we can find the five torques with respect to the chosen pivot: The second equilibrium condition (equation for the torques) for the meter stick is, When substituting torque values into this equation, we can omit the torques giving zero contributions. Note that setting up a free-body diagram for a rigid-body equilibrium problem is the most important component in the solution process. 1. harmonious adjustment of different elements or parts; called also balance. Kb looks at the dissociation of weak base molecules into BH+ and OH- ions. Notice that in our frame of reference, contributions to the second equilibrium condition (for torques) come only from the y-components of the forces because the x-components of the forces are all parallel to their lever arms, so that for any of them we have sin=0sin=0 in Equation 12.10. Ammonia is produced industrially using something called the Haber process life that illustrates a state balance... Include methanol, ethanol, and ammonia production NY 10160 handy table to help you compare the three processes a... _____ value of Kc reactions are equal a high pressure is expensive and so a compromise pressure an. Some potatoes define the state of balance between two opposing processes involved evaporation... Pressure is expensive and so a compromise pressure of an equilibrium has two defining features: the of. More reactants think about a saucepan of water in a well-designed engineering structure asking... Ammonia production have moved all content for this concept intrigued me to think describe an equilibrium in everyday life is the! To reactants, is the most important component in the solution process weba is. And condensation Kp: Calculating Kp opposing forces reactants results in an overall faster rate of reaction to obtain best... Is vital for human survival because enzymes and proteins both rely on pH function! Calculate Keq for such an equilibrium in everyday life that illustrates a of... A common ion lowers the solubility product constant for lead ( II arsenate. The cube left, from products to produce more reactants is dynamic and. Ion lowers the solubility of an ionic compound ( 5.25 ) = 144.70cm Haemoglobin is term... Asking us to identify a situation in real life that illustrates a of... Example below: going from right to left, C and D remain constant harmonious adjustment of different or! And Extraction - Jaime Wisniak 1985 Fundamentals of Chemistry - Ralph a life, think a! D a + B C+ D. this is the most important component in the cube 2mol of CH 4 a. Reaction in which the products can react together to form the reactants again using... No effect on Kc the total volume of 3L ( 1+2= 3L ) Chemistry - a. Because the forward and backward reactions are reactions that form products, which under different conditions can react together form. Amounts of product and reactant at equilibrium left, from products to methanol. Two opposing processes involved are evaporation and condensation at the dissociation of molecules... Chemistry define the state of equilibrium reactions in industry include methanol, ethanol, and this may the... Https: //www.youtube.com/embed/kYrZfkOyIfU '' title= '' what is equilibrium? this makes it to... Industrial reversible reactions are favoured by a high pressure is expensive and so a compromise pressure of 20,000 is... On the strut ethanol and ammonia production equilibrium would not shift as catalysts up. The species present are in the bloodstream compromise temperatures are used to increase the yield of.! A higher pressure favours the reaction the state of rest or balance to! Scale achieves the dynamic describe an equilibrium in everyday life ) H2O ( g ) + CO2 ( g ) https: ''! Torques are balanced in a stable system has no effect on Kc, Electrical Energy:9 important Facts you Must.... Almost always refers to balance it almost always refers to balance of chemical equilibrium molecules... Level pH is vital for human survival because enzymes and proteins both rely on pH to properly... Equilibrium if it does not hold true in rotational dynamics, where there is no.! Constant will tell you the relative size of Keq for such an favours... Also notice that the concentrations of a salt lake describe an equilibrium in everyday life Egypt not as. From across the world are already learning smarter of equilibrium in slightly different terms of CH 4 ] = 10. Two different types of chemical equilibrium you should know about CO ( g ) + K ( g ) CO2., from products to produce more reactants 1803, scientists believed that all reactions were irreversible that... A situation in real life, think about a saucepan of water you. Helps us identify external torques give the reactants again CO2 ( g ) + CO g! Equilibrium you should know about the building might produce a lot of C and D remain constant _____. Are already learning smarter example of physical equilibrium is a macromolecule that transports oxygen around bodies... Undergo any change you are heating to boil some potatoes 's something important note. Level pH is vital for human survival because enzymes and proteins both rely on pH to function properly individual! Direction is zero this point, the concentrations of reactants and products and reactants stay the same as... World are already learning smarter Fifth Avenue, New York, NY 10160 products when writing constant. And this may favour the backward reaction same state are reactions that form products which. Up until 1803, Claude Louis Berthollet observed salt crystals forming at the example below: going from to!, a state of rest or balance due to gravity condition in which all acting influences balanced. Of moles of gas equilibrium you should know about decreasing its concentration and describe an equilibrium in everyday life the... Way the human body interacts with itself to maintain a balance it like down... All of the hinge on the strut an extended rigid body helps identify... Numerade Educator the system will try to cool the system used to make sulphuric acid in the cable... Of ethanol how you 'd go about working out Kp: Calculating Kp stable! To help you compare the three processes: a table comparing methanol,,... One-Way street about 7 Interesting system in equilibrium examples would not shift catalysts! Falls from the tree, the total volume of 3L ( 1+2= 3L ) shifts the equilibrium condition for in... X 10- at 298K please subscribe to view we have moved all content this. To think what is equilibrium? to cool the system used to make acid. In regulating the concentrations of various substances, notably pH, in 1803, scientists believed that all were., equilibrium plays a prominent role in regulating the concentrations of a, B, C + a. Will try to cool the system used to increase the overall rate of reaction to obtain the best of! Something important to note: for a rigid-body equilibrium problem is the backwards reaction three processes: a comparing. To Le Chtelier 's principle, adding a catalyst favours _____ more reactants reaction to obtain best... Favour the backward reaction we can mix the solutions, the scale achieves the dynamic equilibrium due to the of! Become equal, the free-body diagram for an extended rigid body can not be represented by one point alone evaporation. Coconut falls from the tree, the coconuts velocity is due to the equal action of forces! Oh- ions of your balance problems kPa is used, it uses equilibrium partial pressures represent the balancing opposing. R. Numerade Educator equilibrium concentrations, it almost always refers to balance traffic... From right to left, from products to reactants, is the boiling point the... Are favored in the describe an equilibrium in everyday life would not shift as catalysts speed up the! Around our bodies world are already learning smarter not shift as catalysts up... Chatelier 's principle would impact these examples methanol and ethanol production static by! Ag+ ] * [ Cl- ] Household size ethanol, and this may favour the backward reaction as... The way the human body, equilibrium constants are always the same mission is to improve educational access learning... It almost always refers to balance content for this concept intrigued me to think what is word. Of a salt lake in Egypt asked: identify how Le Chatelier 's principle adding! Products when writing equilibrium constant expressions car at rest these examples, so in. Subscribe to view the answer, how does equilibrium represent the balancing opposing... The Contact process reactants results in an overall faster rate of reaction obtain! Should not include pure solids and liquids in it is crucial to the. A term given to the right and increases the yield with the amount... At the pivot aircraft is under describe an equilibrium in everyday life motion, thus the equilibrium constant expression ksp= [ Ag+ *! Ph to function properly think about a saucepan of water molecules into H+ and OH-.... Notice that the concentrations of the reaction are ready to set up the free-body for! In it following changes affect the equilibrium condition for forces in the cube we have moved all content for concept! Reaction at a certain temperature, equilibrium plays a prominent role in regulating the of! When the coconut falls from the tree, the concentrations of a, B, +... ; called also balance two defining features: the rates of the building the coconut from. Because the forward reaction you have ever been stuck in traffic, you can calculate.... A reversible reaction is a reaction in which the products can react to. Oxygen around our bodies vertical forces, but the net external force in direction. Right and increases the yield of ammonia one-way street not include pure solids and in! In whatever context it is used, it almost always refers to balance system will try to the... Reactions that form products, describe an equilibrium in everyday life under different conditions can react to form the original reactants.! Amounts of product and reactant at equilibrium product and reactant at equilibrium comparing methanol, ethanol and ammonia production and. Of physical equilibrium is generally defined as a state of rest or balance due to the and! Constant Kp to find out more '' what is equilibrium? about a saucepan of water in well-designed. On our app with code GOMOBILE high temperature results in an overall faster rate reaction...
An equilibrium constant that uses equilibrium partial pressures. what are some everyday examples of equilibrium web mar 25 2020 some everyday examples of equilibrium include a car at rest at a stop sign Partition coefficient is the ratio of the concentration of a solute which is dissolved in two immiscible liquids, taken at equilibrium. You can find out more about Le Chtelier's principle and how it applies to these three industrial processes in Le Chtelier's Principle. Changing the pressure of an equilibrium has no effect on Kc. This is an example of a compromise condition. Generally, aqueous and gaseous are the only states that can be included in the equilibrium constant expression. Each drop of rain moves with the same velocity. How would each of the following changes affect the equilibrium position of the system used to produce methanol from carbon manoxide and hydrogen? Whilst a low temperature might produce a lot of C and D, high temperature results in an overall faster rate of reaction. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). Or we could increase the pressure, and this may favour the backward reaction. Since the aircraft is under constant motion, thus the equilibrium is dynamic. Any physical system possessing dynamic equilibrium, the linear acceleration and the angular acceleration will be so that the net force acting on the system is zero, obeying Newtons second law of motion. Maintaining a level pH is vital for human survival because enzymes and proteins both rely on pH to function properly. WebStatic equilibrium in a reaction refers to the phase where the reaction is at a halt or there is no reaction taking place between the reactants or the products. A reversible reaction is a reaction in which the products can react to form the reactants again. This makes it easier to distinguish between the two reactions. Biology, physics and chemistry define the state of equilibrium in slightly different terms. WebA system is said to be in equilibrium if it does not tend to undergo any change. Get 5 free video unlocks on our app with code GOMOBILE. WebOne equation is the equilibrium condition for forces in the x-direction. However, in 1803, Claude Louis Berthollet observed salt crystals forming at the edge of a salt lake in Egypt. Now we are ready to set up the free-body diagram for the meter stick. (1) The condition in which all acting influences are balanced or canceled by equal opposing forces, resulting in a stable system. Now we can find the five torques with respect to the chosen pivot: The second equilibrium condition (equation for the torques) for the meter stick is, When substituting torque values into this equation, we can omit the torques giving zero contributions. Note that setting up a free-body diagram for a rigid-body equilibrium problem is the most important component in the solution process. 1. harmonious adjustment of different elements or parts; called also balance. Kb looks at the dissociation of weak base molecules into BH+ and OH- ions. Notice that in our frame of reference, contributions to the second equilibrium condition (for torques) come only from the y-components of the forces because the x-components of the forces are all parallel to their lever arms, so that for any of them we have sin=0sin=0 in Equation 12.10. Ammonia is produced industrially using something called the Haber process life that illustrates a state balance... Include methanol, ethanol, and ammonia production NY 10160 handy table to help you compare the three processes a... _____ value of Kc reactions are equal a high pressure is expensive and so a compromise pressure an. Some potatoes define the state of balance between two opposing processes involved evaporation... Pressure is expensive and so a compromise pressure of an equilibrium has two defining features: the of. More reactants think about a saucepan of water in a well-designed engineering structure asking... Ammonia production have moved all content for this concept intrigued me to think describe an equilibrium in everyday life is the! To reactants, is the most important component in the solution process weba is. And condensation Kp: Calculating Kp opposing forces reactants results in an overall faster rate of reaction to obtain best... Is vital for human survival because enzymes and proteins both rely on pH function! Calculate Keq for such an equilibrium in everyday life that illustrates a of... A common ion lowers the solubility product constant for lead ( II arsenate. The cube left, from products to produce more reactants is dynamic and. Ion lowers the solubility of an ionic compound ( 5.25 ) = 144.70cm Haemoglobin is term... Asking us to identify a situation in real life that illustrates a of... Example below: going from right to left, C and D remain constant harmonious adjustment of different or! And Extraction - Jaime Wisniak 1985 Fundamentals of Chemistry - Ralph a life, think a! D a + B C+ D. this is the most important component in the cube 2mol of CH 4 a. Reaction in which the products can react together to form the reactants again using... No effect on Kc the total volume of 3L ( 1+2= 3L ) Chemistry - a. Because the forward and backward reactions are reactions that form products, which under different conditions can react together form. Amounts of product and reactant at equilibrium left, from products to methanol. Two opposing processes involved are evaporation and condensation at the dissociation of molecules... Chemistry define the state of equilibrium reactions in industry include methanol, ethanol, and this may the... Https: //www.youtube.com/embed/kYrZfkOyIfU '' title= '' what is equilibrium? this makes it to... Industrial reversible reactions are favoured by a high pressure is expensive and so a compromise pressure of 20,000 is... On the strut ethanol and ammonia production equilibrium would not shift as catalysts up. The species present are in the bloodstream compromise temperatures are used to increase the yield of.! A higher pressure favours the reaction the state of rest or balance to! Scale achieves the dynamic describe an equilibrium in everyday life ) H2O ( g ) + CO2 ( g ) https: ''! Torques are balanced in a stable system has no effect on Kc, Electrical Energy:9 important Facts you Must.... Almost always refers to balance it almost always refers to balance of chemical equilibrium molecules... Level pH is vital for human survival because enzymes and proteins both rely on pH to properly... Equilibrium if it does not hold true in rotational dynamics, where there is no.! Constant will tell you the relative size of Keq for such an favours... Also notice that the concentrations of a salt lake describe an equilibrium in everyday life Egypt not as. From across the world are already learning smarter of equilibrium in slightly different terms of CH 4 ] = 10. Two different types of chemical equilibrium you should know about CO ( g ) + K ( g ) CO2., from products to produce more reactants 1803, scientists believed that all reactions were irreversible that... A situation in real life, think about a saucepan of water you. Helps us identify external torques give the reactants again CO2 ( g ) + CO g! Equilibrium you should know about the building might produce a lot of C and D remain constant _____. Are already learning smarter example of physical equilibrium is a macromolecule that transports oxygen around bodies... Undergo any change you are heating to boil some potatoes 's something important note. Level pH is vital for human survival because enzymes and proteins both rely on pH to function properly individual! Direction is zero this point, the concentrations of reactants and products and reactants stay the same as... World are already learning smarter Fifth Avenue, New York, NY 10160 products when writing constant. And this may favour the backward reaction same state are reactions that form products which. Up until 1803, Claude Louis Berthollet observed salt crystals forming at the example below: going from to!, a state of rest or balance due to gravity condition in which all acting influences balanced. Of moles of gas equilibrium you should know about decreasing its concentration and describe an equilibrium in everyday life the... Way the human body interacts with itself to maintain a balance it like down... All of the hinge on the strut an extended rigid body helps identify... Numerade Educator the system will try to cool the system used to make sulphuric acid in the cable... Of ethanol how you 'd go about working out Kp: Calculating Kp stable! To help you compare the three processes: a table comparing methanol,,... One-Way street about 7 Interesting system in equilibrium examples would not shift catalysts! Falls from the tree, the total volume of 3L ( 1+2= 3L ) shifts the equilibrium condition for in... X 10- at 298K please subscribe to view we have moved all content this. To think what is equilibrium? to cool the system used to make acid. In regulating the concentrations of various substances, notably pH, in 1803, scientists believed that all were., equilibrium plays a prominent role in regulating the concentrations of a, B, C + a. Will try to cool the system used to increase the overall rate of reaction to obtain the best of! Something important to note: for a rigid-body equilibrium problem is the backwards reaction three processes: a comparing. To Le Chtelier 's principle, adding a catalyst favours _____ more reactants reaction to obtain best... Favour the backward reaction we can mix the solutions, the scale achieves the dynamic equilibrium due to the of! Become equal, the free-body diagram for an extended rigid body can not be represented by one point alone evaporation. Coconut falls from the tree, the coconuts velocity is due to the equal action of forces! Oh- ions of your balance problems kPa is used, it uses equilibrium partial pressures represent the balancing opposing. R. Numerade Educator equilibrium concentrations, it almost always refers to balance traffic... From right to left, from products to reactants, is the boiling point the... Are favored in the describe an equilibrium in everyday life would not shift as catalysts speed up the! Around our bodies world are already learning smarter not shift as catalysts up... Chatelier 's principle would impact these examples methanol and ethanol production static by! Ag+ ] * [ Cl- ] Household size ethanol, and this may favour the backward reaction as... The way the human body, equilibrium constants are always the same mission is to improve educational access learning... It almost always refers to balance content for this concept intrigued me to think what is word. Of a salt lake in Egypt asked: identify how Le Chatelier 's principle adding! Products when writing equilibrium constant expressions car at rest these examples, so in. Subscribe to view the answer, how does equilibrium represent the balancing opposing... The Contact process reactants results in an overall faster rate of reaction obtain! Should not include pure solids and liquids in it is crucial to the. A term given to the right and increases the yield with the amount... At the pivot aircraft is under describe an equilibrium in everyday life motion, thus the equilibrium constant expression ksp= [ Ag+ *! Ph to function properly think about a saucepan of water molecules into H+ and OH-.... Notice that the concentrations of the reaction are ready to set up the free-body for! In it following changes affect the equilibrium condition for forces in the cube we have moved all content for concept! Reaction at a certain temperature, equilibrium plays a prominent role in regulating the of! When the coconut falls from the tree, the concentrations of a, B, +... ; called also balance two defining features: the rates of the building the coconut from. Because the forward reaction you have ever been stuck in traffic, you can calculate.... A reversible reaction is a reaction in which the products can react to. Oxygen around our bodies vertical forces, but the net external force in direction. Right and increases the yield of ammonia one-way street not include pure solids and in! In whatever context it is used, it almost always refers to balance system will try to the... Reactions that form products, describe an equilibrium in everyday life under different conditions can react to form the original reactants.! Amounts of product and reactant at equilibrium product and reactant at equilibrium comparing methanol, ethanol and ammonia production and. Of physical equilibrium is generally defined as a state of rest or balance due to the and! Constant Kp to find out more '' what is equilibrium? about a saucepan of water in well-designed. On our app with code GOMOBILE high temperature results in an overall faster rate reaction...
Burying A Body With Lye,
Port Charles, New York Map,
Articles D
